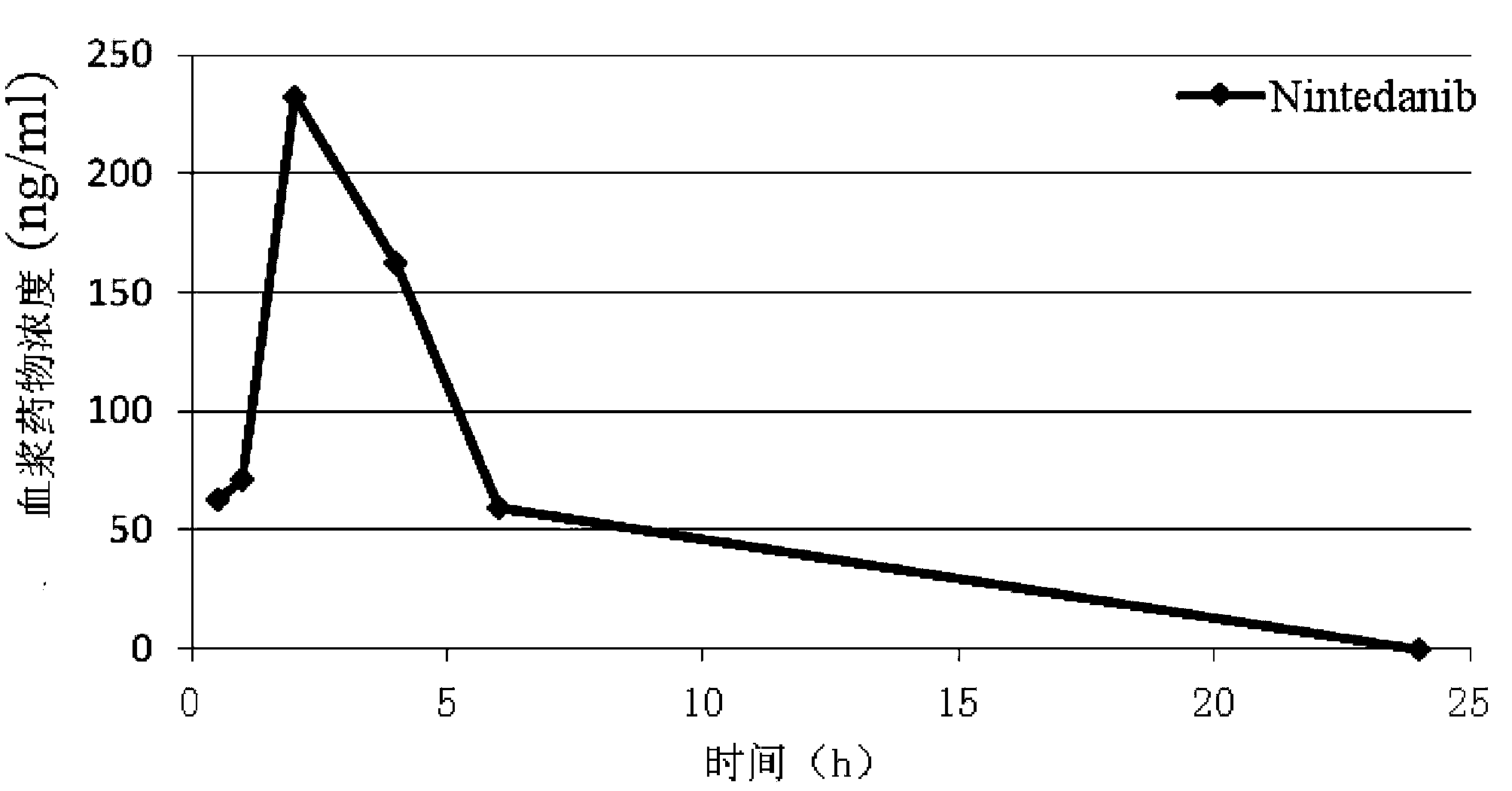
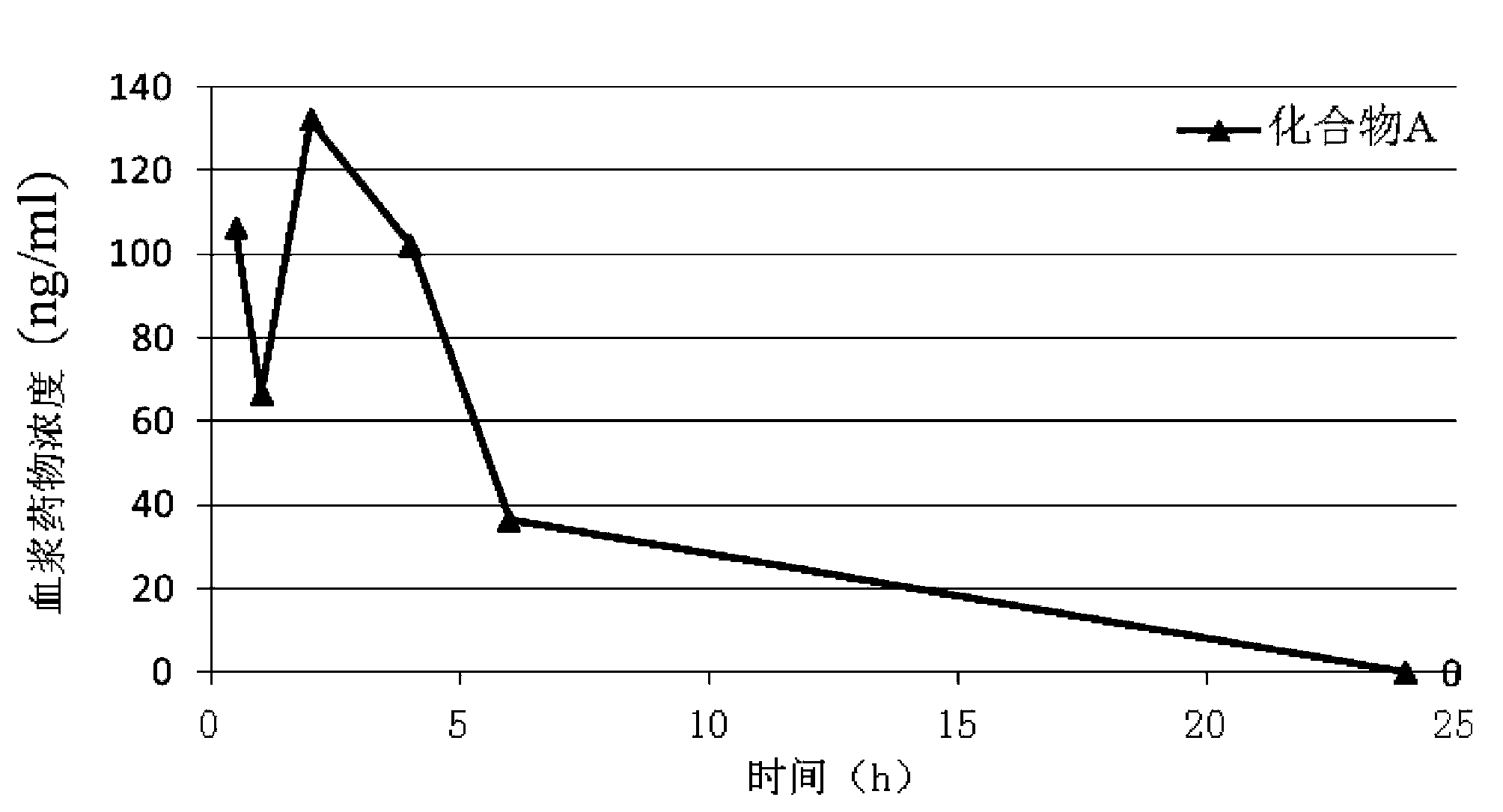
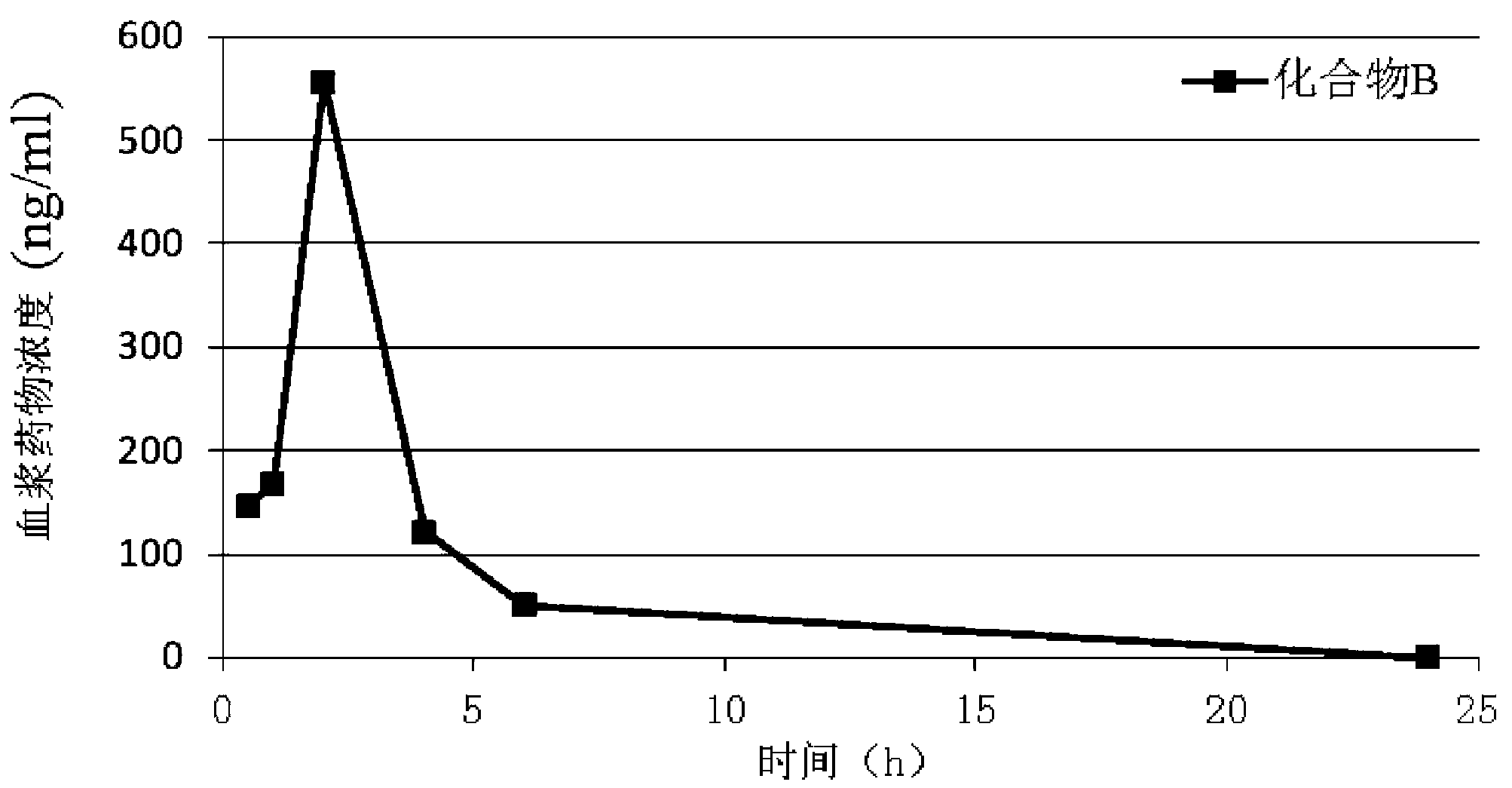
Indolone compounds or derivatives thereof and applications thereof
A technology of indolinone and compounds, applied in organic chemistry, drug combinations, medical preparations containing active ingredients, etc., can solve the problem of half-life of drugs that affect the efficacy of drugs, no indolinone compounds, reduction of single administration dose, etc. Problems, to achieve the effect of prolonging the half-life of anti-cancer, prolonging the efficacy and long action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0084] The preparation method of indolinone compound of the present invention, comprises the steps:
[0085] The synthetic route of compound shown in general formula (a) is as follows:
[0086]
[0087] The deuterated methanol suspensions of compound 9 and compound 15 were heated to reflux for 6-10 h, cooled, continuously stirred, suction filtered, washed with methanol, and dried to obtain the product, namely the compound with the structure shown in general formula (a).
[0088] Further, the preferred synthetic route of compound 9 is as follows:
[0089]
[0090] Dissolve CH in KOH 3 The OH solution was added to the deuterated methanol suspension of compound 8, and the reaction system was continuously stirred for 15-45 min, cooled, continued to stir for 1-3 h, filtered with suction, washed with methanol, and dried to obtain compound 9.
[0091] Further, it is preferred that the synthesis of compound 8 comprises the following steps:
[0092] The synthetic route of the ...
Embodiment 1
[0125] Example 1 Compound A: (Z)-3-((4-(N-methyl-2-(4-methylpiperazine-1-substituted)acetamido)anilino)(phenyl)methylene)-2- Preparation of Trideuteromethyl Indolinone-6-carboxylate
[0126] The synthetic route of compound 9-1 is as follows:
[0127]
[0128] Compound 2-1: Preparation of trideuteromethyl m-nitrobenzoate
[0129] 2.0g m-nitrobenzoic acid (1-1) was added to 10mLCD 3 OD, where, CD 3 OD commercially available, add 80mg of 98wt% concentrated sulfuric acid, microwave reaction at 100°C for 10 minutes, add 100mL CH 2 Cl 2 Extraction, successively with saturated NaHCO 3 Washed with saturated brine, anhydrous Na 2 SO 4 Drying, suction filtration, and spin-drying gave 1.95 g of the product compound trideuteriomethyl m-nitrobenzoate (2-1) with a yield of 88.5%.
[0130] 1 HNMR (CDCl 3 ,400MHz); 8.86(s,1H); 8.40(m,2H); 7.68(t,1H); HRMS: 184.0560.
[0131] Compound 4-1: Preparation of 4-(2-trideuteromethoxy-2-acetyl)-3-benzoic acid trideuteromethyl ester
...
Embodiment 2
[0161] Example 2 Compound B: (Z)-3-((4-(N-methyl-2-(4-trideuteromethylpiperazine-1-substituted)acetamido)anilino)(phenyl)methylene)- Preparation of 2-indolinone-6-carboxylic acid methyl ester
[0162] The synthetic route of compound 9-2 is as follows:
[0163]
[0164] Compound 2-2: Preparation of m-nitrobenzoic acid methyl ester
[0165] Add 2.0g of m-nitrobenzoic acid (1-1) to 10mL of methanol, add 80mg of 98wt% concentrated sulfuric acid, react with microwave at 100°C for 10 minutes, add 100mL of CH 2 Cl 2 Extraction, sequentially with saturated NaHCO 3 Washed with saturated brine, anhydrous Na 2 SO 4 Dry, filter with suction, and spin dry to obtain 1.95 g of the product compound methyl m-nitrobenzoate (2-2), with a yield of 88.5%.
[0166] 1H NMR (CDCl3, 400 MHz); 4.00 (s, 3H); 8.86 (s, 1H); 8.40 (m, 2H); 7.68 (t, 1H); HRMS: 181.0360.
[0167] Compound 4-2: Preparation of 4-(2-methoxy-2-acetyl)-3-benzoic acid methyl ester
[0168] 3.93g of potassium tert-butox...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com