Calcitriol soft capsule preparation production method
A technology of calcitriol and soft capsules, which is used in capsule delivery, bone diseases, pharmaceutical formulations, etc., can solve the problems of long-term stable existence, light, heat and air sensitivity, and low bioavailability, so as to improve comprehensive performance. and market competitiveness, short half-life, eliminating the effect of absorbing excipients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1, a kind of preparation method of calcitriol soft capsule
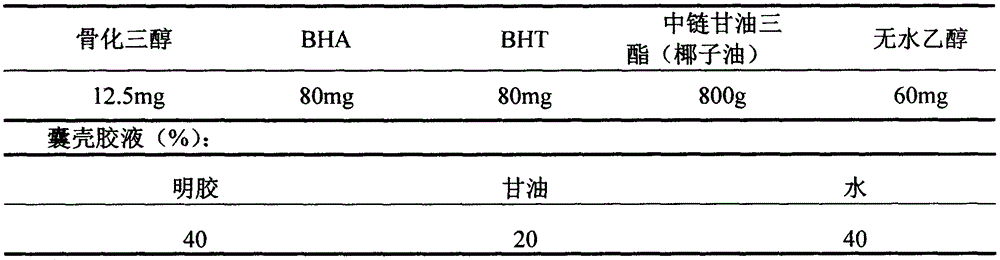
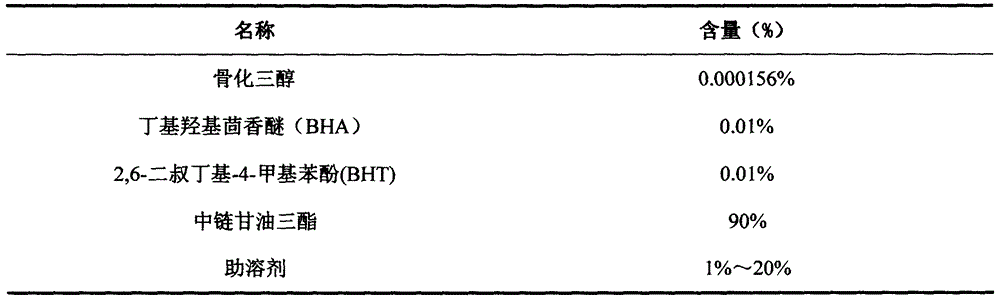
[0027] Contents:
[0028]
[0029] Preparation Process:
[0030] (1) Place the above-mentioned prescription amount of material in the batching tank, stir and mix for 30 minutes; grind it into a homogeneous mixture and mix it evenly to form a uniform and stable content and vacuumize it for later use;
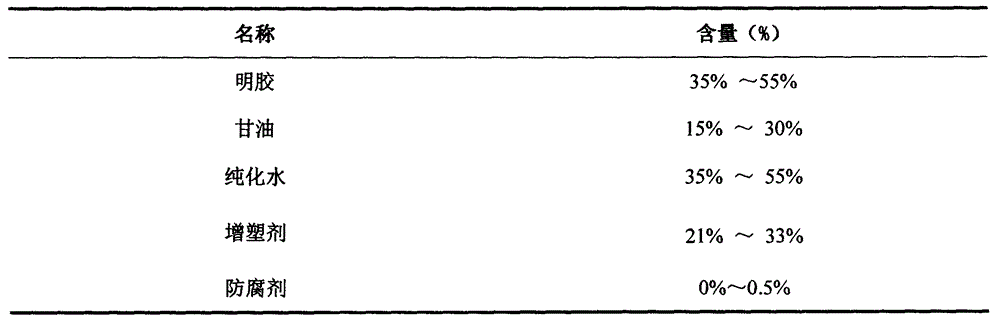
[0031] (2) Put 20 parts by weight of glycerin and 40 parts by weight of purified water into the plastic tank, heat and stir to raise the temperature to 70-80°C, then put in 40 parts by weight of gelatin, and continue stirring for 20-30 minutes;
[0032] (3) Vacuumize to remove air bubbles, filter while it is hot, then discharge and cool to 60-65°C, keep warm;
[0033] (4) Put the content obtained in the above step (1) and the glue solution obtained in step (2) respectively into a rotary molded soft capsule machine for pill pressing, and the rotational speed of the rotary molded soft capsule machin...
Embodiment 2
[0037] Embodiment two, the stability experiment of calcitriol soft capsule of the present invention:
[0038] Influencing factor test: take calcitriol soft capsules, respectively at 60 ℃, 25 ℃ and relative humidity 92.5%, illuminance 4500Ix 3 kinds of conditions for investigation. The result is that the samples are easy to absorb water and become soft under high humidity conditions, but under light conditions It is easy to harden, and its disintegration time limit and content have no obvious change. Accelerated test: Take 3 batches of samples under the market packaging conditions, place them for 12 months under the conditions of temperature (40±2) qC and relative humidity (75±10)%. , 9, 12 months, samples were taken for stability investigation. As a result, the appearance, disintegration time limit and content of the samples did not change significantly. Long-term test: 3 batches of samples were taken under the market packaging conditions, and 36 samples were placed at room te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com