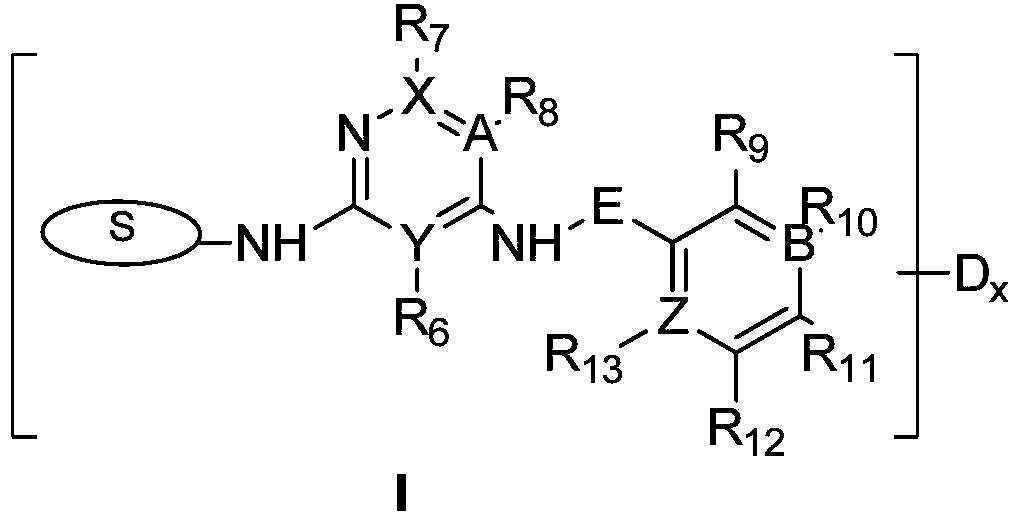
FAK inhibitor and combined medicine thereof
A technology of solvates and compounds, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
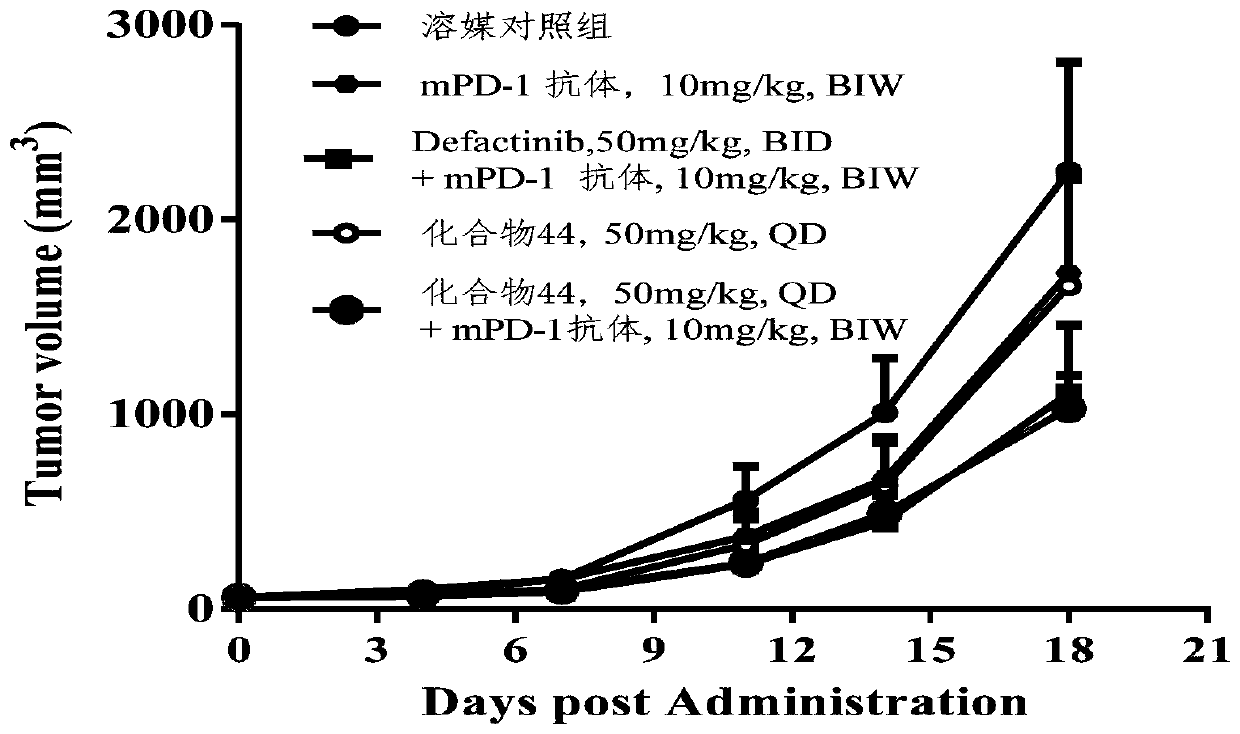
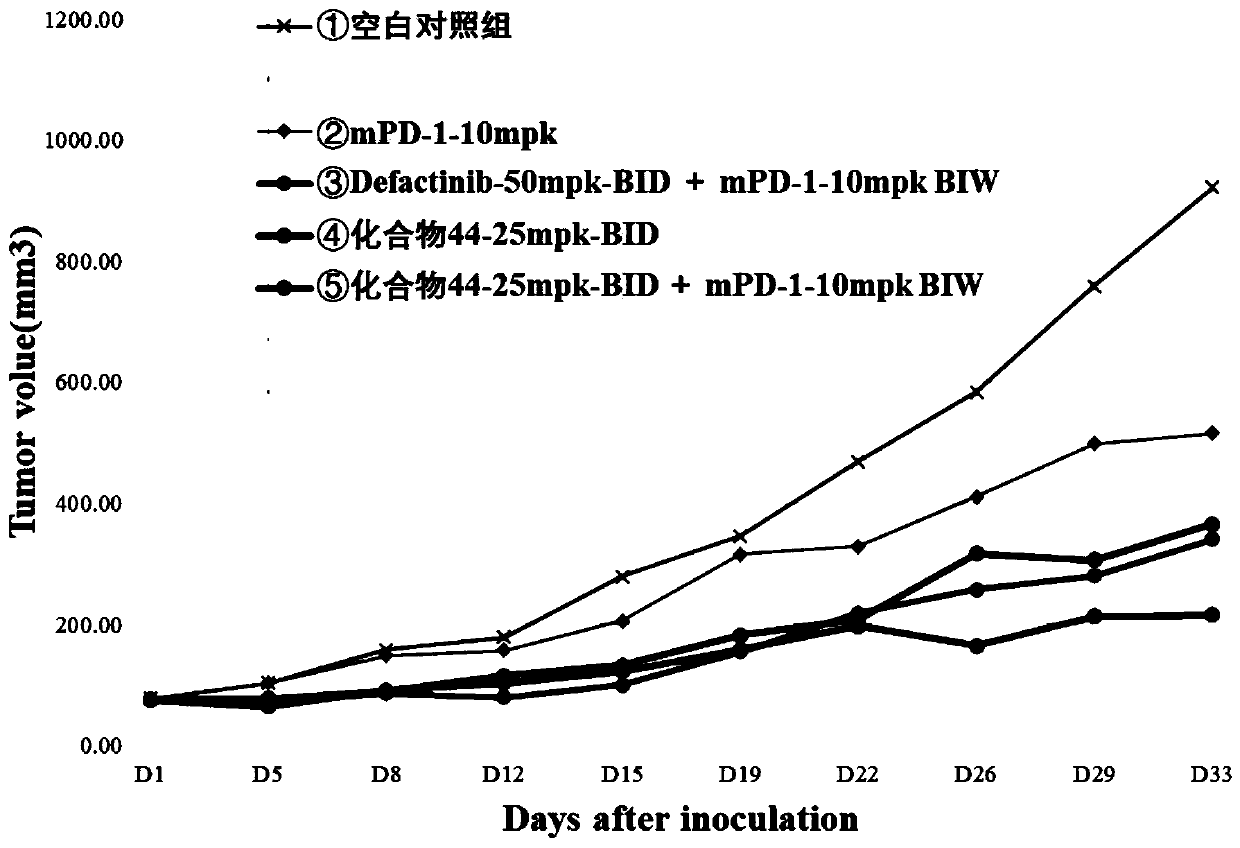
Image
Examples
Embodiment 1
[0091] Embodiment 1, synthesis of N-trideuteromethyl-4-((4-(((3-(N-methylmethylsulfonamido)pyrazin-2-yl)methyl)amino)-5-( Trifluoromethyl)pyrimidin-2-yl)amino)benzoic acid amide (compound 25)
[0092]
[0093] The first step: synthesis of compound 25-2
[0094] 25-1 (200mg, 0.39mmol), DMAP (1.29g, 10.57mmol) was added to 10mL of dichloromethane, and then (Boc) 2 O (1.71 g, 7.83 mmol). The system was refluxed in an oil bath for 24h. The next day, cool to room temperature, add dichloromethane and 0.1N HCl solution, extract, leave to stand and separate layers, the organic phase is washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent is removed by rotary evaporation after suction filtration. The crude product, 25-2, was obtained as an off-white solid, 136 mg, yield: 42.8%. MS (M+1): 811.2.
[0095] The second step: synthesis of compound 25-3
[0096] Add 25-2 (136 mg, 0.17 mmol), deuterated methylamine hydrochloride (189 mg, 2.68 mmol) into 5 ...
Embodiment 2
[0101] Embodiment 2, synthesis of N-methyl-4-((4-(((3-(N-trideuteromethylmethanesulfonamido)pyrazin-2-yl)methyl)amino)-5-( Trifluoromethyl)pyrimidin-2-yl)amino)benzoic acid amide (Compound 41)
[0102]
[0103] The first step: Synthesis of N-trideuteromethylmethanesulfonamide (compound 41-1)
[0104] Deuteromethylamine hydrochloride (7.75g, 109.99mmol) was placed in a 250mL single-neck round bottom flask, and dichloromethane (120mL) was added at the same time, and stirred at room temperature. Then the system was transferred to an ice-water bath for cooling and stirring. After 15 minutes, triethylamine (21.73 g, 214.75 mmol) and DMAP (128 mg, 1.05 mmol) were added in sequence. After completion, the system was kept in the ice-water bath for 10 minutes. Afterwards, methanesulfonyl chloride (12.0 g, 104.76 mmol) was added to the system. After completion, the ice bath was removed, and the system was stirred and reacted at room temperature overnight. The next day, when the TLC ...
Embodiment 3
[0118] Example 3, synthesis of N-trideuteromethyl-4-((4-(((3-(N-trideuteromethylmethanesulfonamido)pyrazin-2-yl)methyl)amino)- 5-(trifluoromethyl)pyrimidin-2-yl)amino)benzoic acid amide (compound 44) and its hydrochloride
[0119]
[0120] The first step: Synthesis of (4-(trideuteromethylcarbamoyl)phenyl) tert-butyl carbamate (compound 44-1)
[0121] N-Boc-4-aminobenzoic acid (6.0g, 25.29mmol) and EDCI (7.27g, 37.93mmol) were weighed and placed in a 250mL single-neck round bottom flask, and DMF (50mL) was added at the same time, and stirred at room temperature. Subsequently, triethylamine (6.40 g, 63.22 mmol) and deuterated methylamine hydrochloride (1.96 g, 27.82 mmol) were added to the system, and the system was stirred and reacted at room temperature overnight. On the next day, the plate was sampled, and TLC showed that the reaction had ended. Add ethyl acetate (50mL) and water (50mL) to the system and stir vigorously, then let stand to separate the layers, back-extr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com