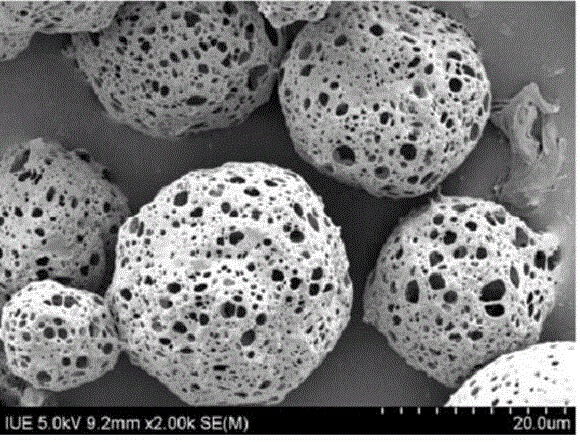
Thymalfasin sustained release micro-sphere preparation and preparing method thereof
A new technology of sustained-release microsphere preparation and thymus method, which can be used in antiviral agents, microcapsules, pharmaceutical formulations, etc., and can solve the problems of intense preparation process, high temperature, and difficulty in separating and purifying products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 200 mg of L-polylactic acid (PLLA) was dissolved in 5 ml of dichloromethane to obtain an oil phase of polylactic acid copolymer, and 3 mg of stabilizer Pronroni F-127 was added. Dissolve 80mg of thymofasin, 15mg of gelatin, and 0.5ml of ammonium bicarbonate in 600ml of water for injection, add the oil phase to the water phase, and use a cell disruptor for ultrasonic emulsification for 1.5min (power 200W, ultrasound 1s, interval 1s ) to form a uniform and stable W / O emulsion. Add the above solution to the newly prepared 1000ml aqueous solution of sodium alginate with a mass fraction of 2%, and use a cell disruptor to ultrasonic emulsify for 1.5min. The ultrasonic emulsification conditions are the same as above to form a uniform and stable W / O / W emulsion. The W / O / W emulsion was immediately put into a syringe, and injected into 500ml of a prepared calcium chloride solution with a mass fraction of 10% through a high-pressure microcapsule forming device, and the emulsion was...
Embodiment 2
[0028] 300 mg of L-polylactic acid (PLLA) was dissolved in 10 ml of ethyl acetate to obtain an oil phase of polylactic acid copolymer, and 3.5 mg of stabilizer Pronroni F-127 was added. Dissolve 150mg of thymofasin, 20mg of trehalose, and 4.0ml of ammonium bicarbonate in 1000ml of water for injection, add the oil phase to the water phase, and use a cell disruptor for ultrasonic emulsification for 1.5min (power 200W, ultrasound 1s, interval 1s) A uniform and stable W / O emulsion is formed. Add the above solution to 1500ml of newly prepared sodium alginate aqueous solution with a mass fraction of 5%, and use a cell disruptor to ultrasonically emulsify for 1.5min. The ultrasonic emulsification conditions are the same as above to form a uniform and stable W / O / W emulsion. The W / O / W emulsion was immediately put into a syringe, and injected into 1000ml of a prepared calcium chloride solution with a mass fraction of 8% through a high-pressure microcapsule forming device, and the emulsi...
Embodiment 3
[0030]400 mg of L-polylactic acid (PLLA) was dissolved in 10 ml of methylene chloride to obtain an oil phase of polylactic acid copolymer, and 4.8 mg of stabilizer Pronroni F-127 was added. Dissolve 100mg of thymofasin, 10mg of trehalose, and 1.0ml of ammonium bicarbonate in 800ml of water for injection, add the oil phase to the water phase, and use a cell disruptor for ultrasonic emulsification for 1.5min (power 200W, ultrasound 1s, interval 1s) A uniform and stable W / O emulsion is formed. Add the above solution to 1200ml of newly prepared sodium alginate aqueous solution with a mass fraction of 5%, and use a cell disruptor to ultrasonically emulsify for 1.5min. The emulsification conditions are the same as above to form a uniform and stable W / O / W emulsion. The prepared W The / O / W emulsion is immediately filled into a syringe, injected into 1200ml of a prepared calcium chloride solution with a mass fraction of 6% through a high-pressure microcapsule forming device, stirred th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com