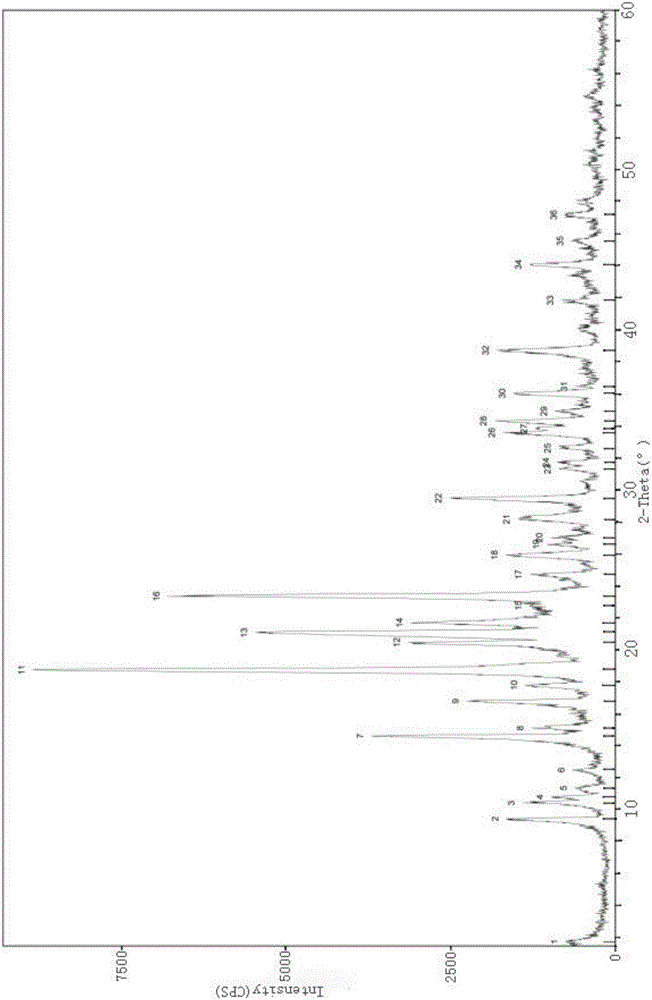
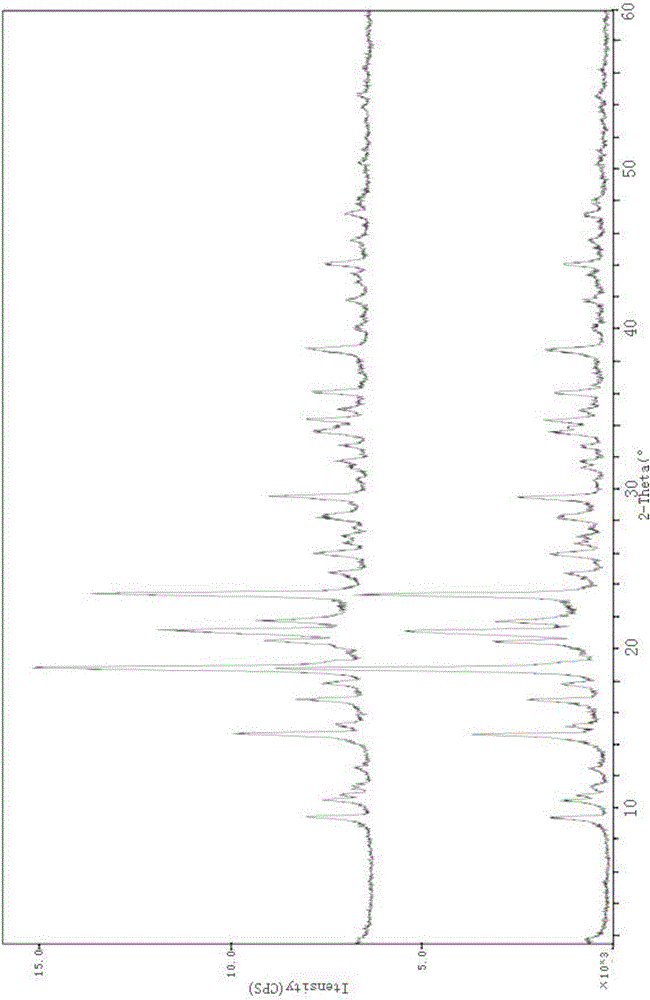
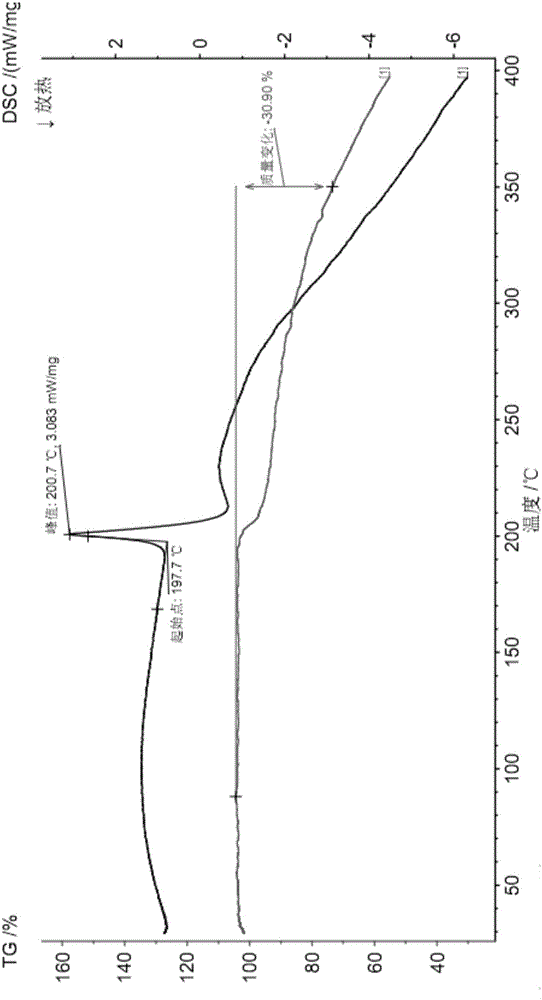
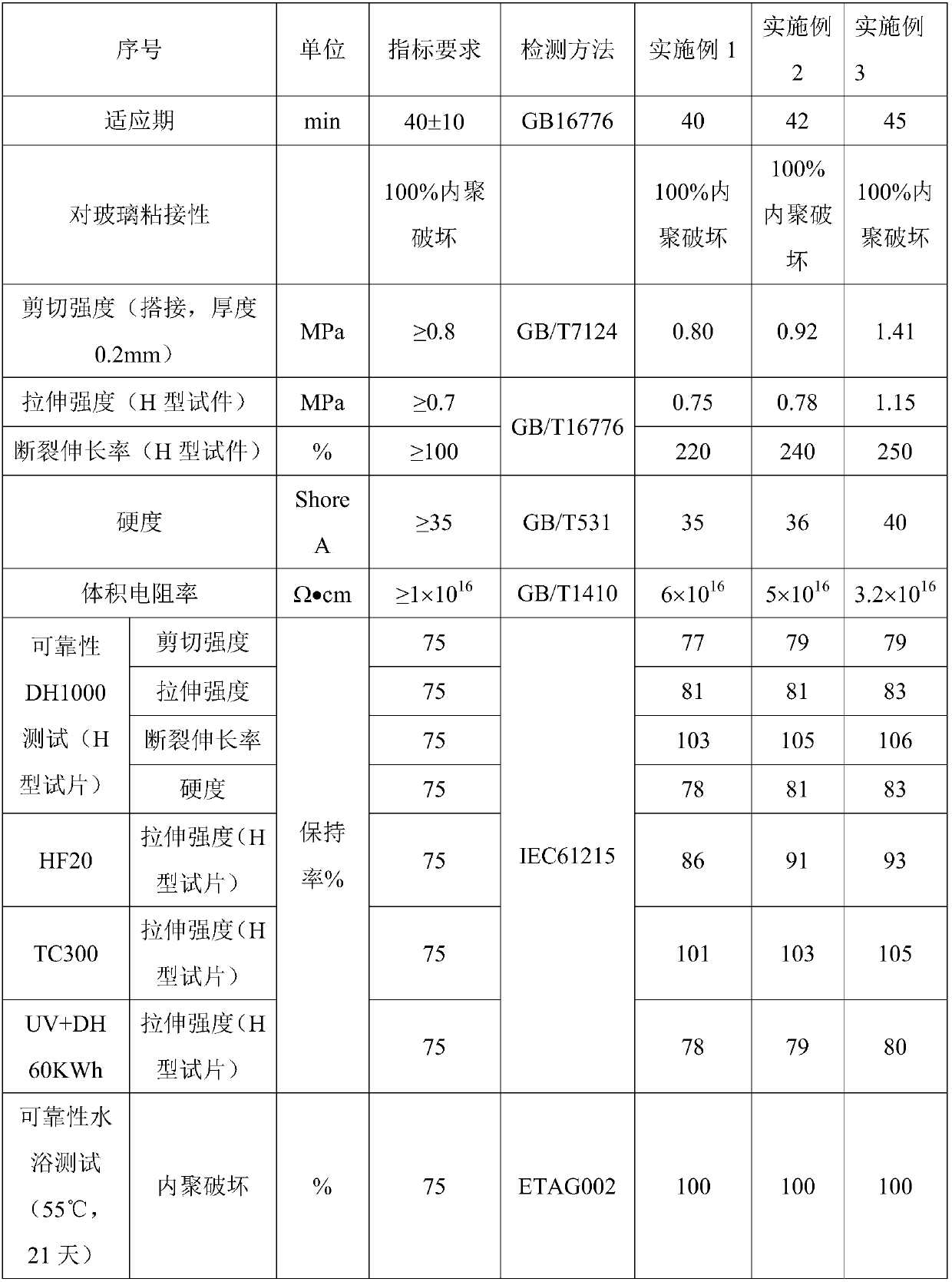
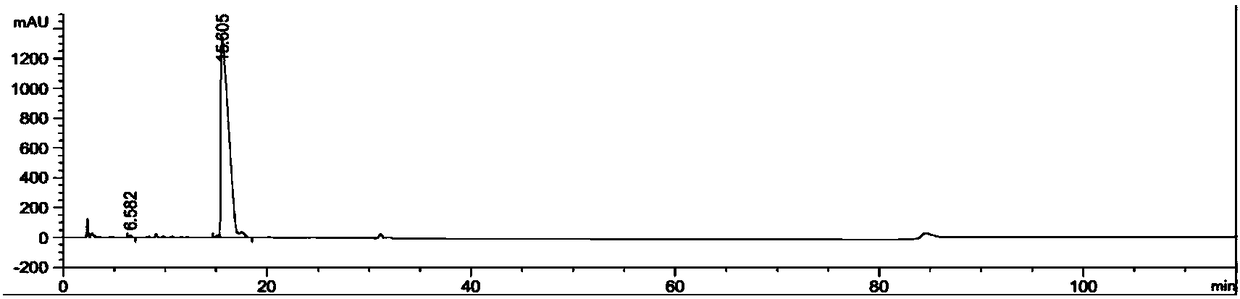
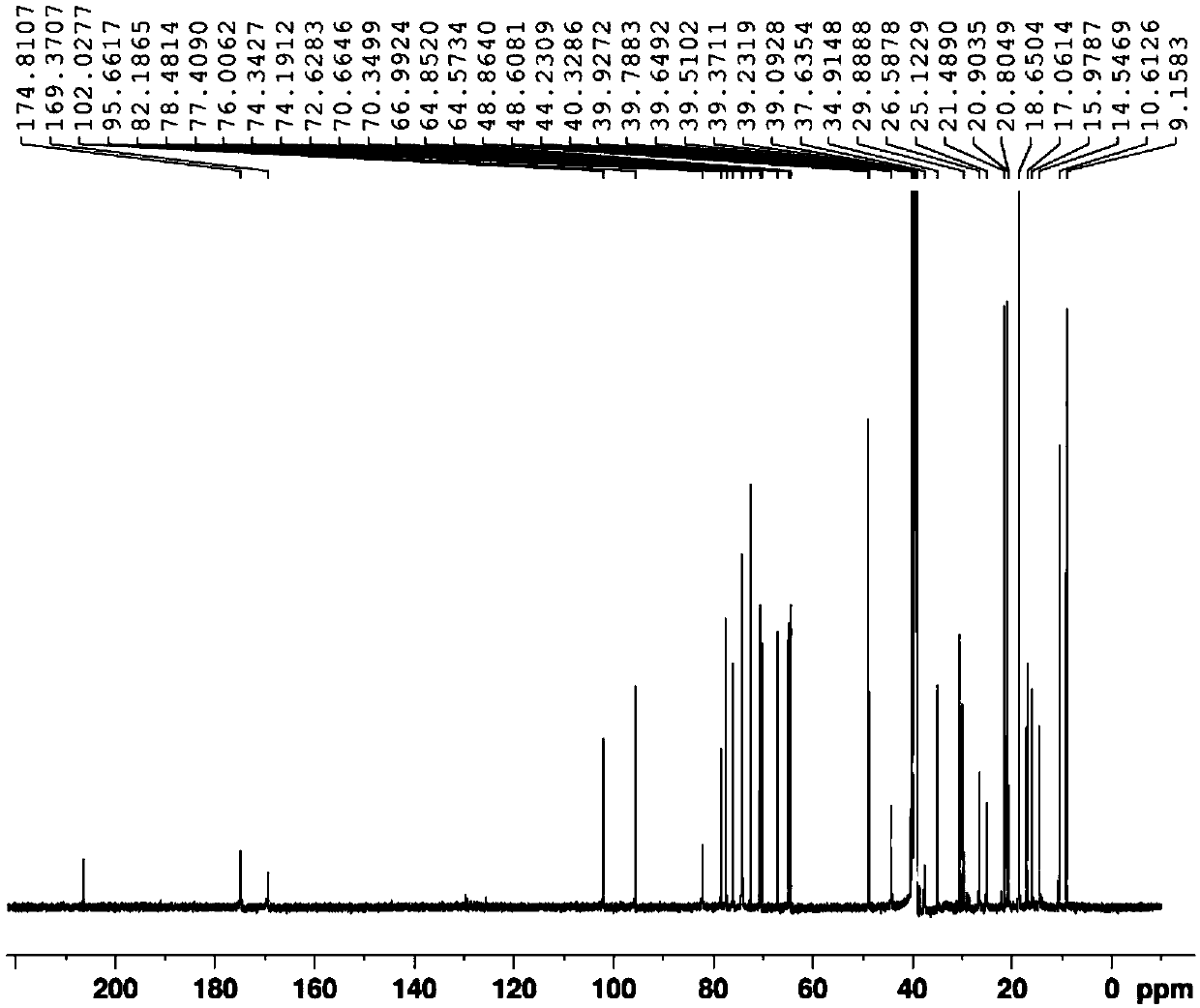
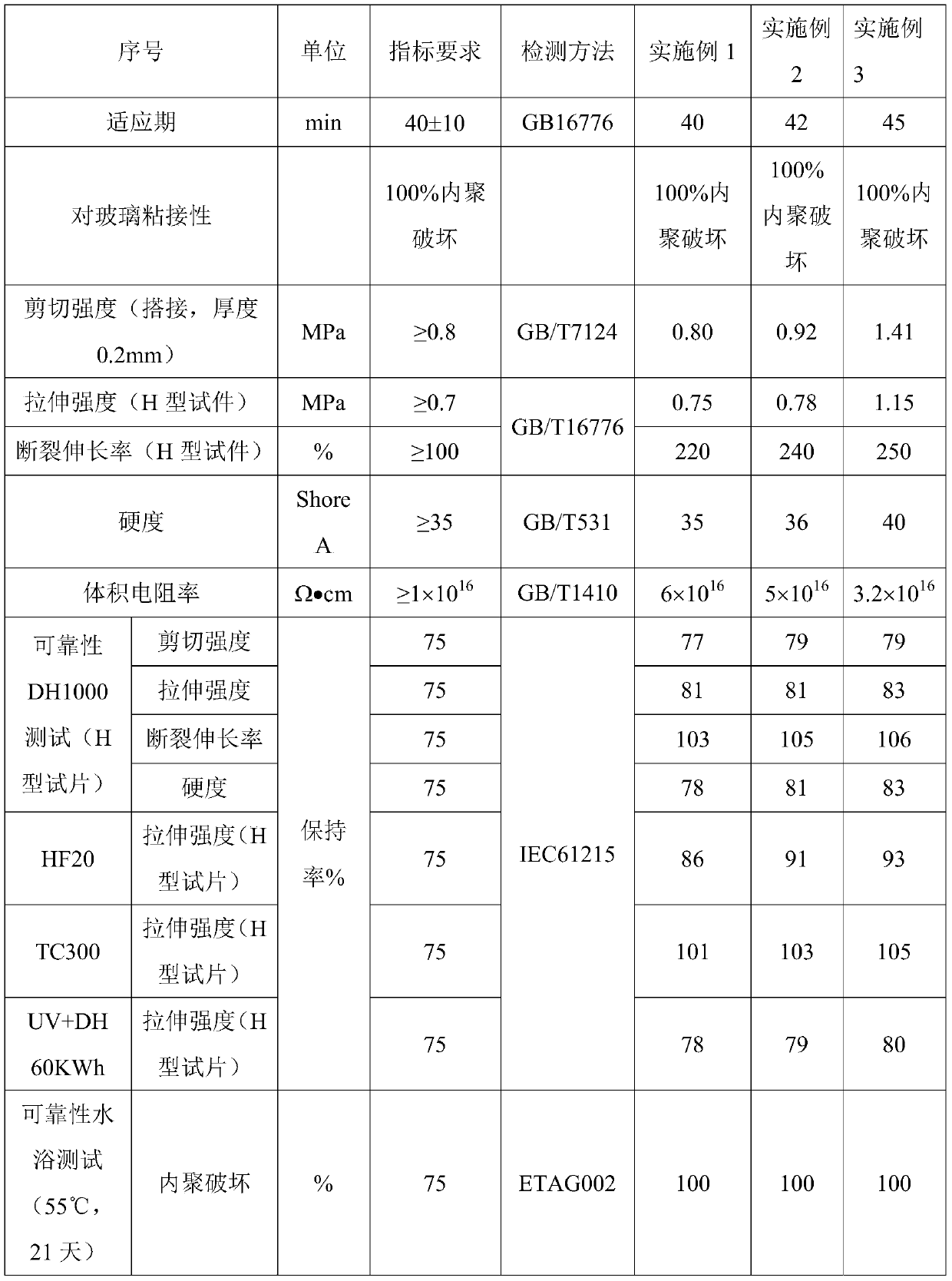
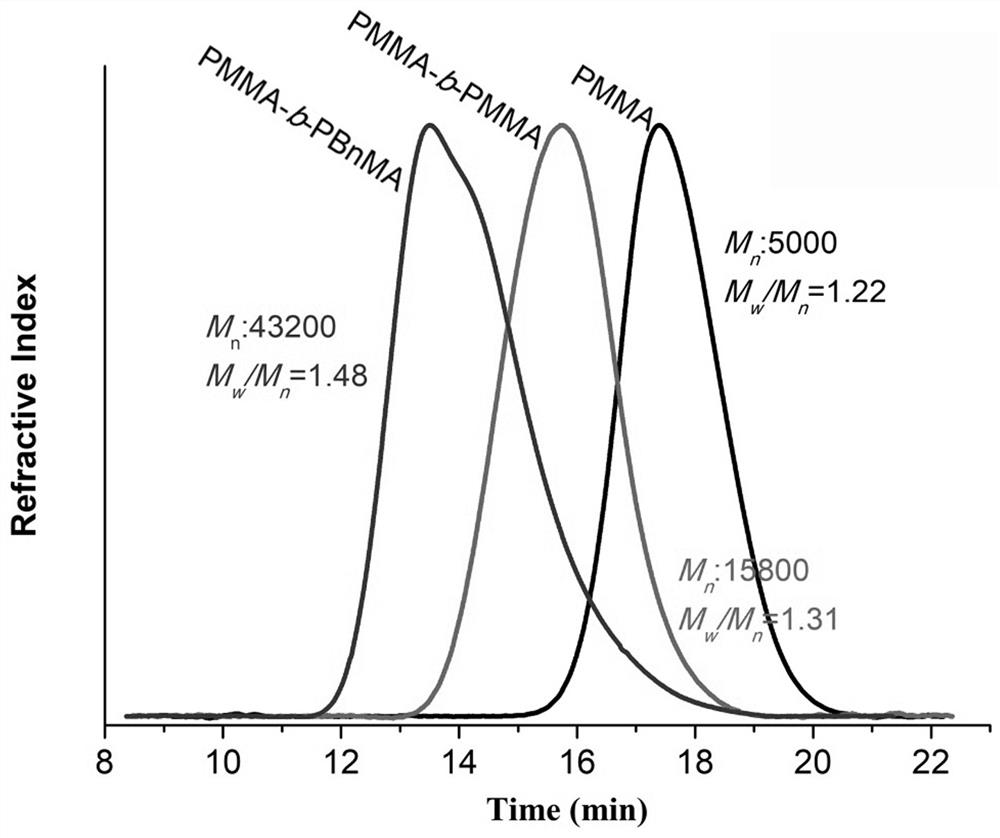
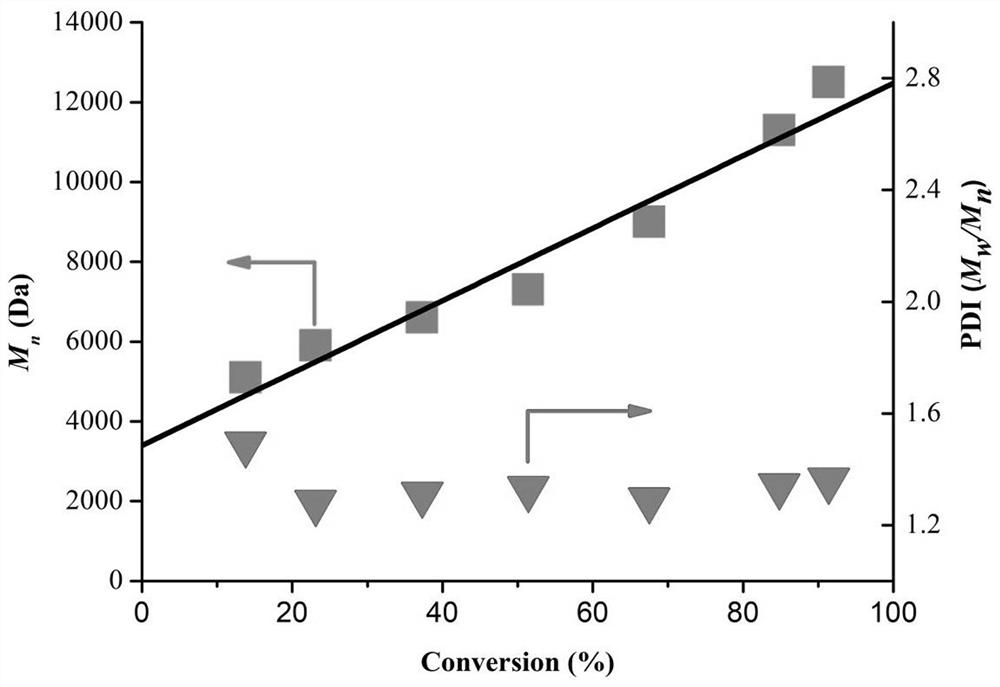
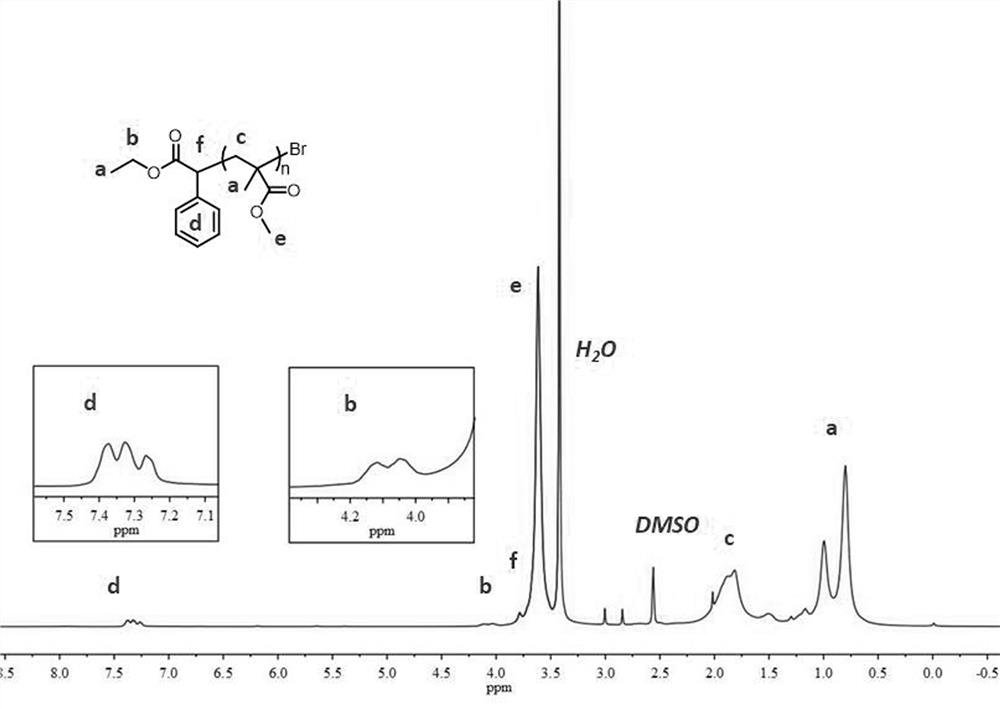
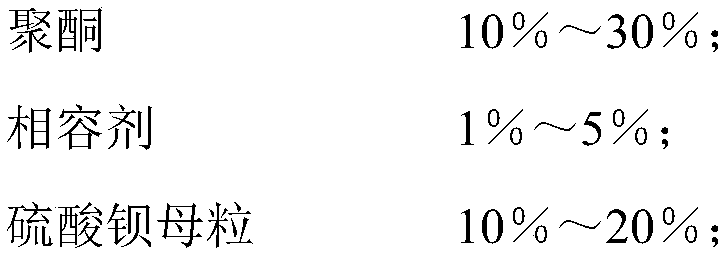Patents
Literature
33results about How to "Avoid degradation reactions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Modified polylactic acid composite material suitable for 3D printing and preparation method of composite material
The invention relates to a modified polylactic acid composite material suitable for 3D printing and a preparation method of the composite material. The modified polylactic acid composite material suitable for 3D printing is prepared from the following raw materials in percentage by weight: 80-90% of polylactic acid, 6-10% of a flexibilizer, 1-2% of a nucleating agent, 1-3% of a dispersing agent, 1-2% of a chain extender and 1-3% of a compatilizer. The preparation method of the modified polylactic acid composite material suitable for 3D printing comprises the following steps: mixing the raw materials by virtue of a high-speed mixer, and extruding and drawing by using a single-screw extruder. Compared with the prior art, the composite material prepared by the method is good in flexibility; meanwhile, the impact strength, the heat resistance and the breakage elongation can be greatly improved; the printing is smooth when the composite material is used for 3D printing; the finished product has the advantages of smooth surface, beautiful and shapely appearance and stable size.
Owner:SHANGHAI RES INST OF MATERIALS CO LTD
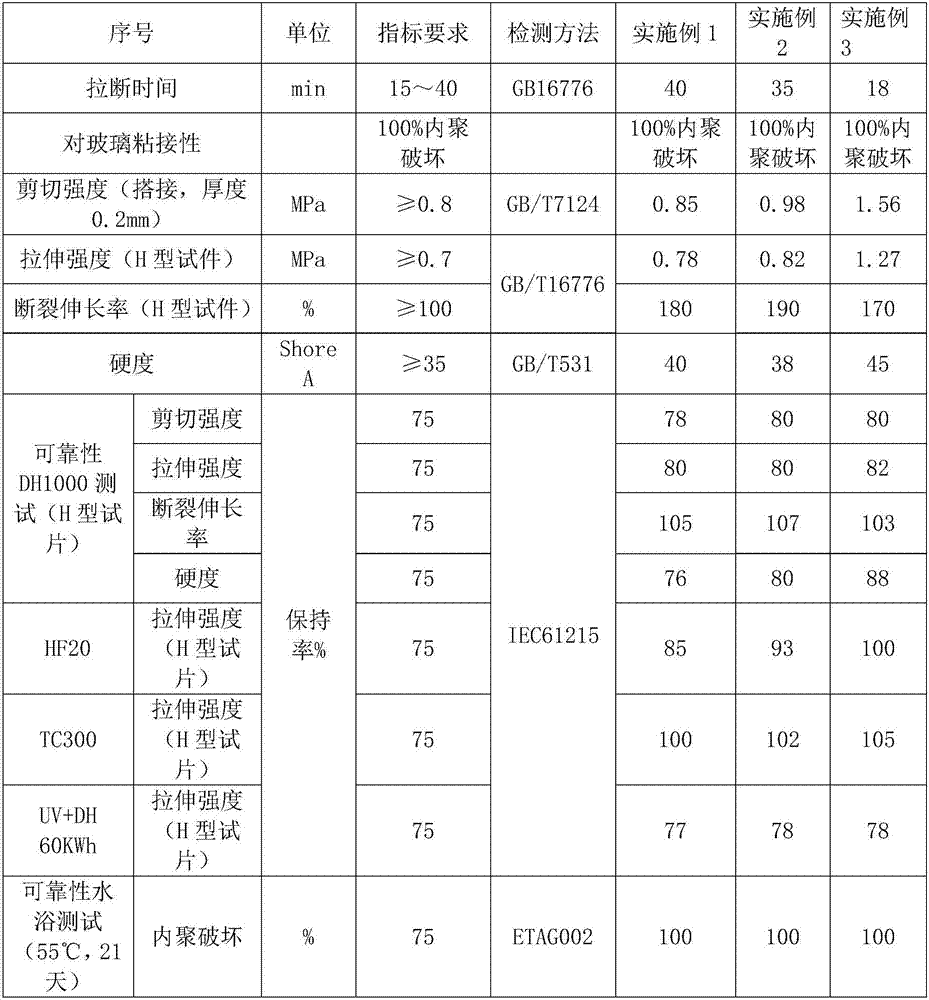
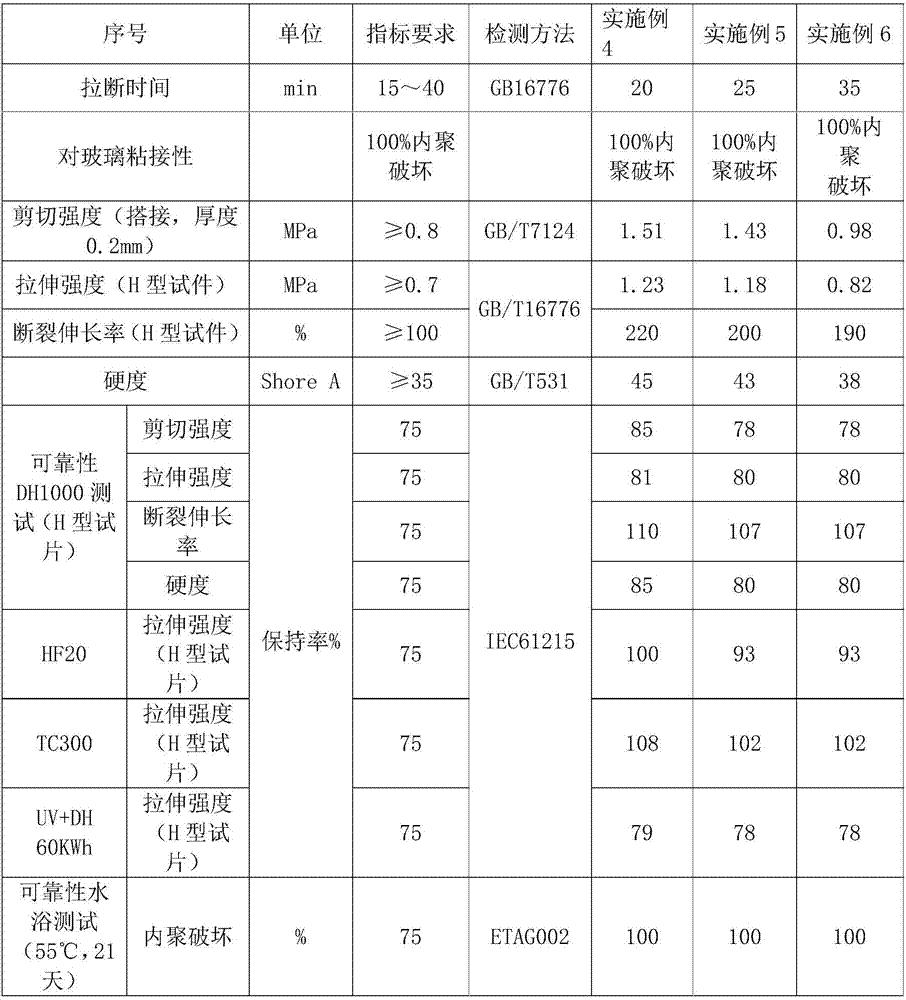
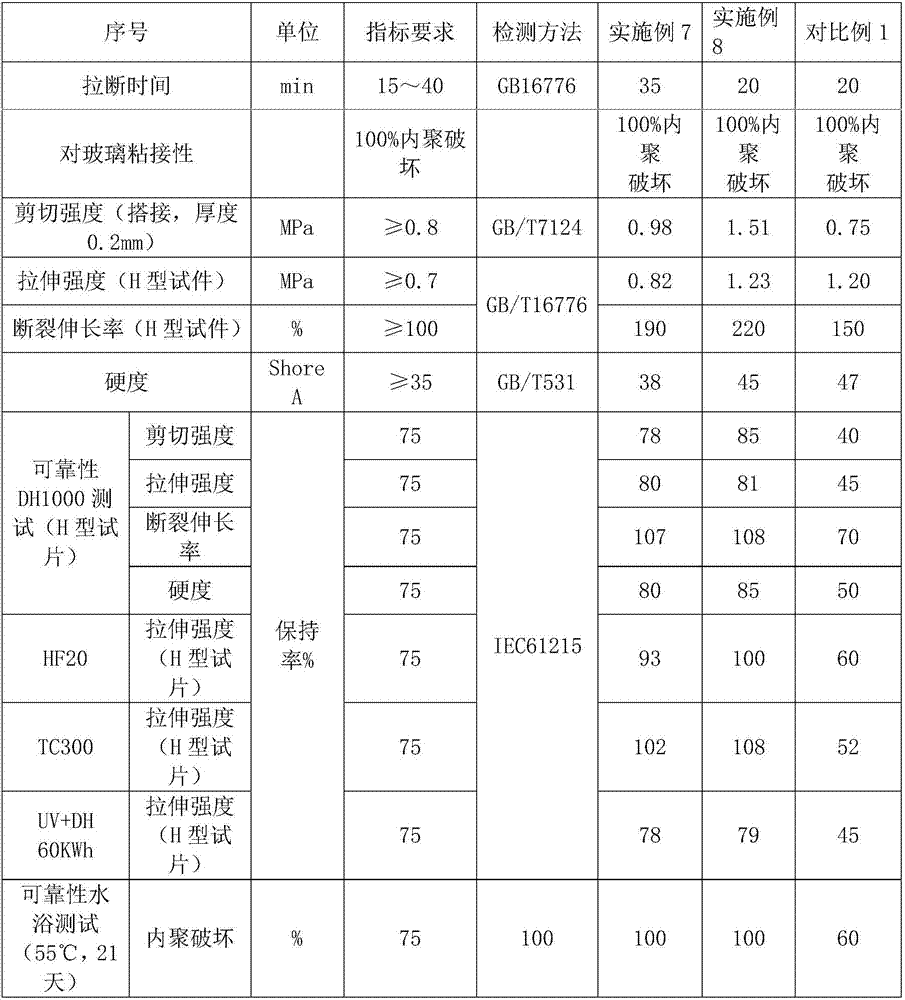
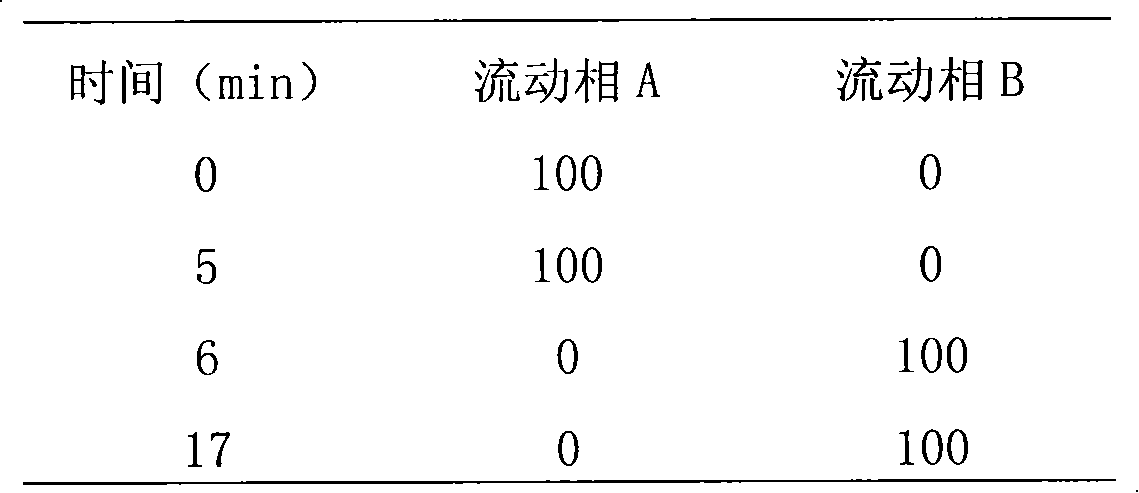
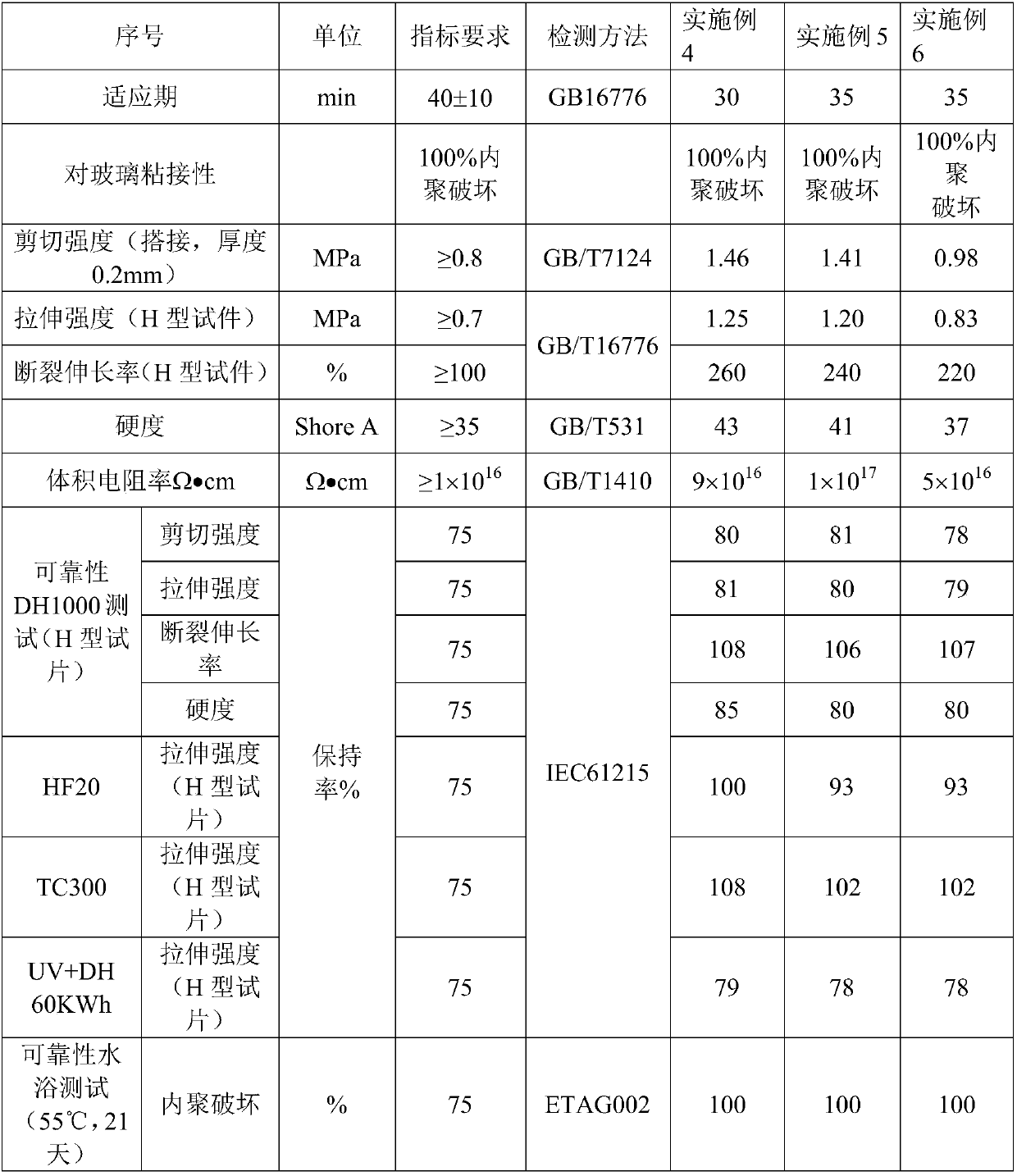
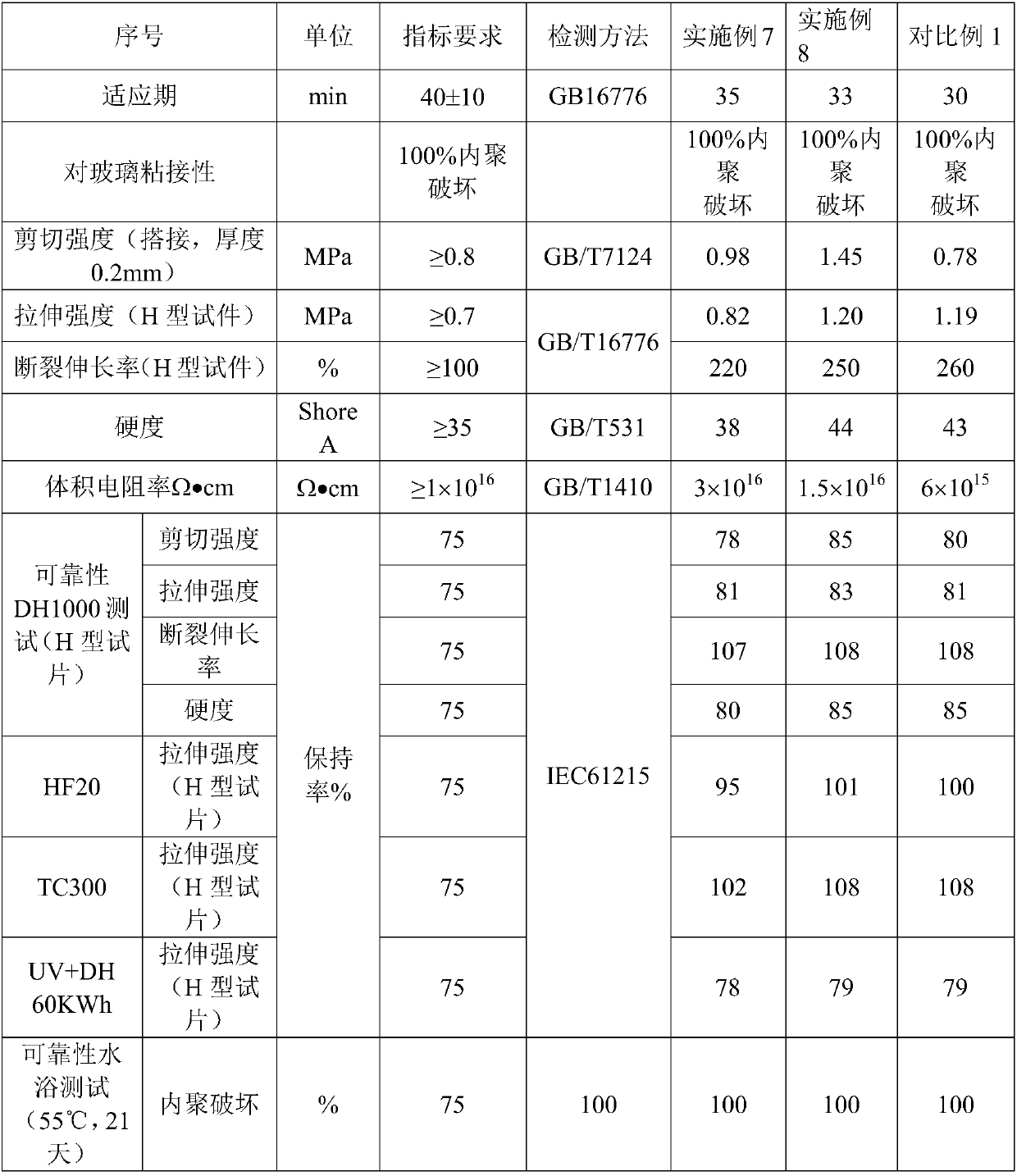
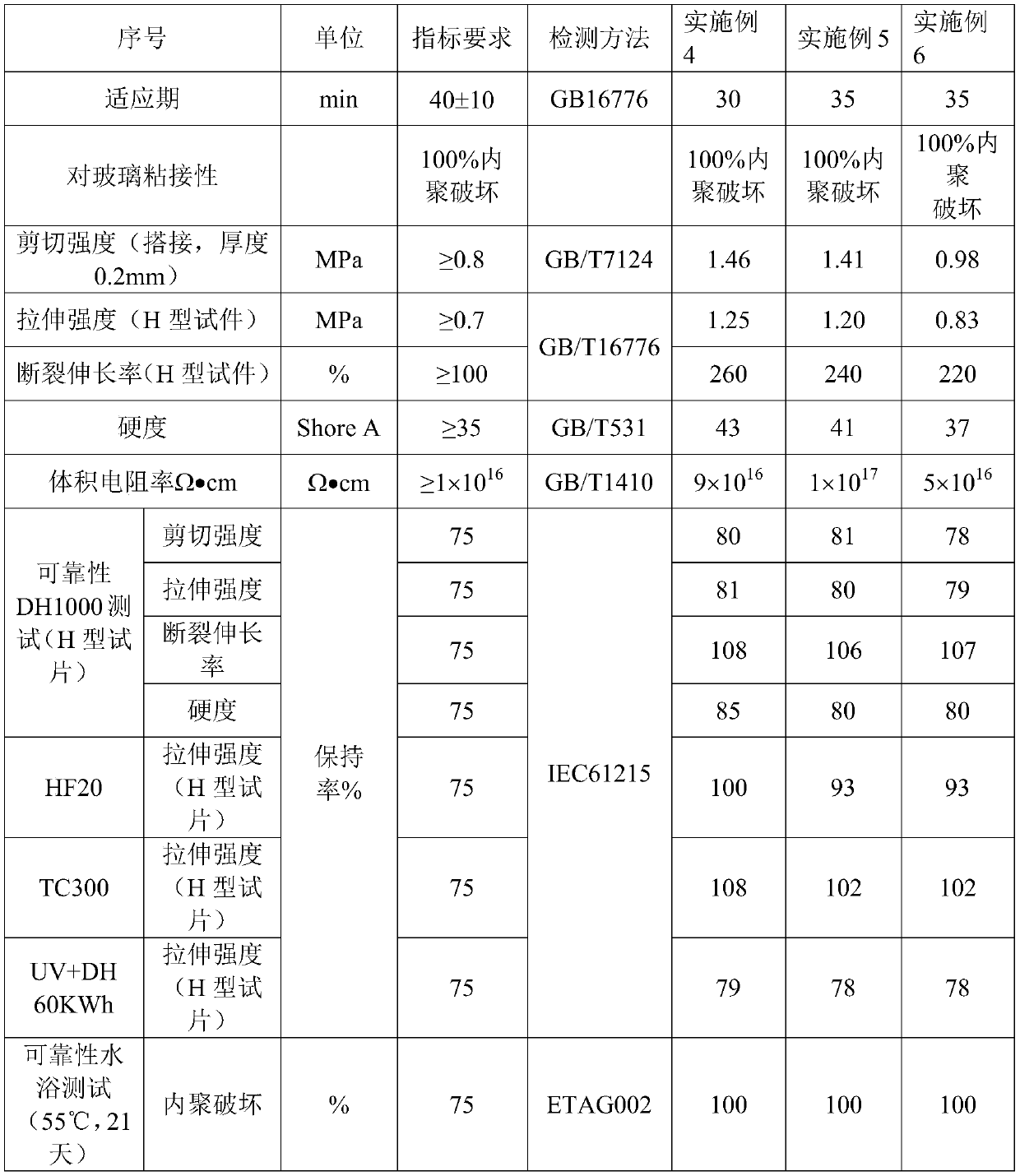
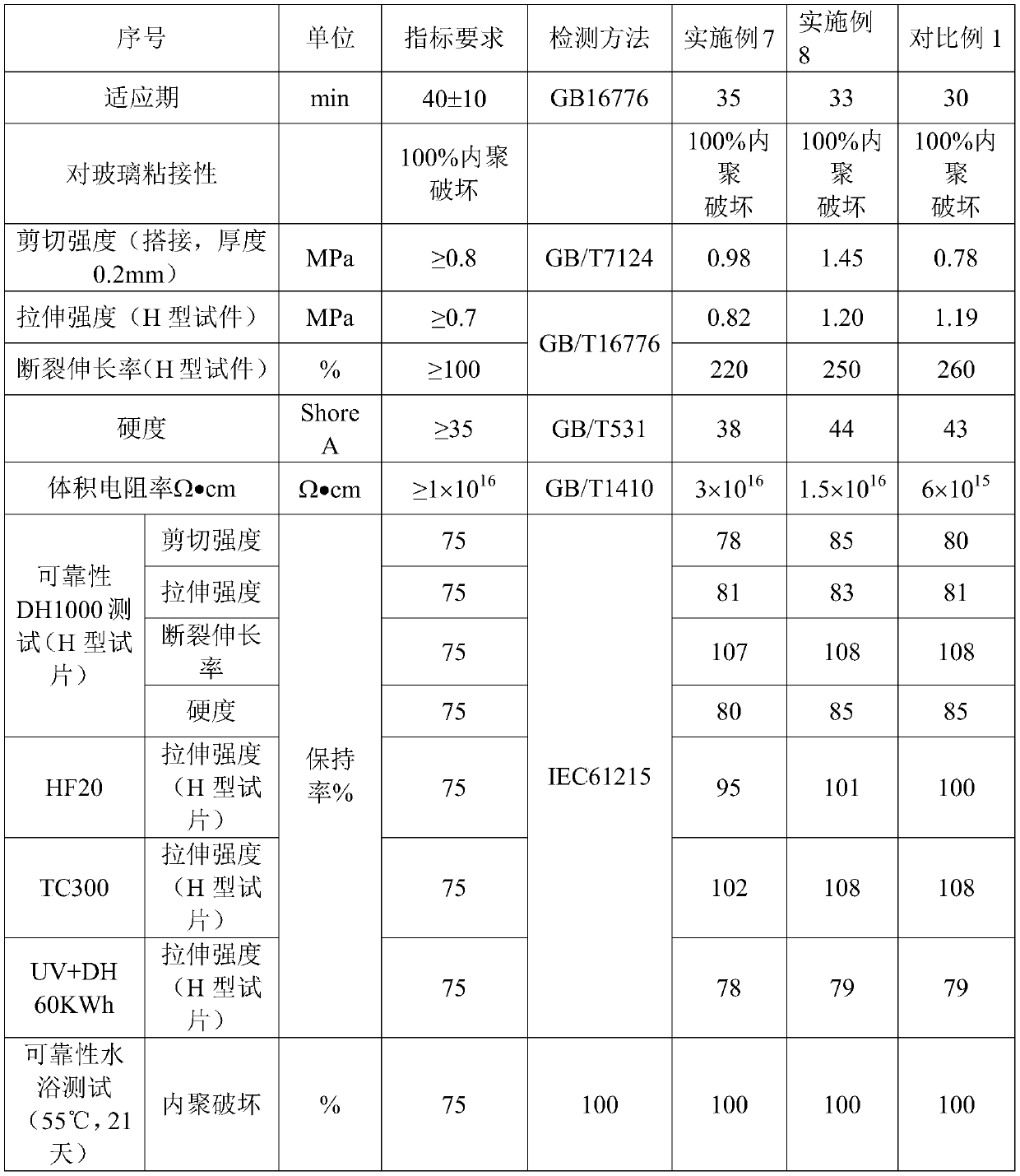
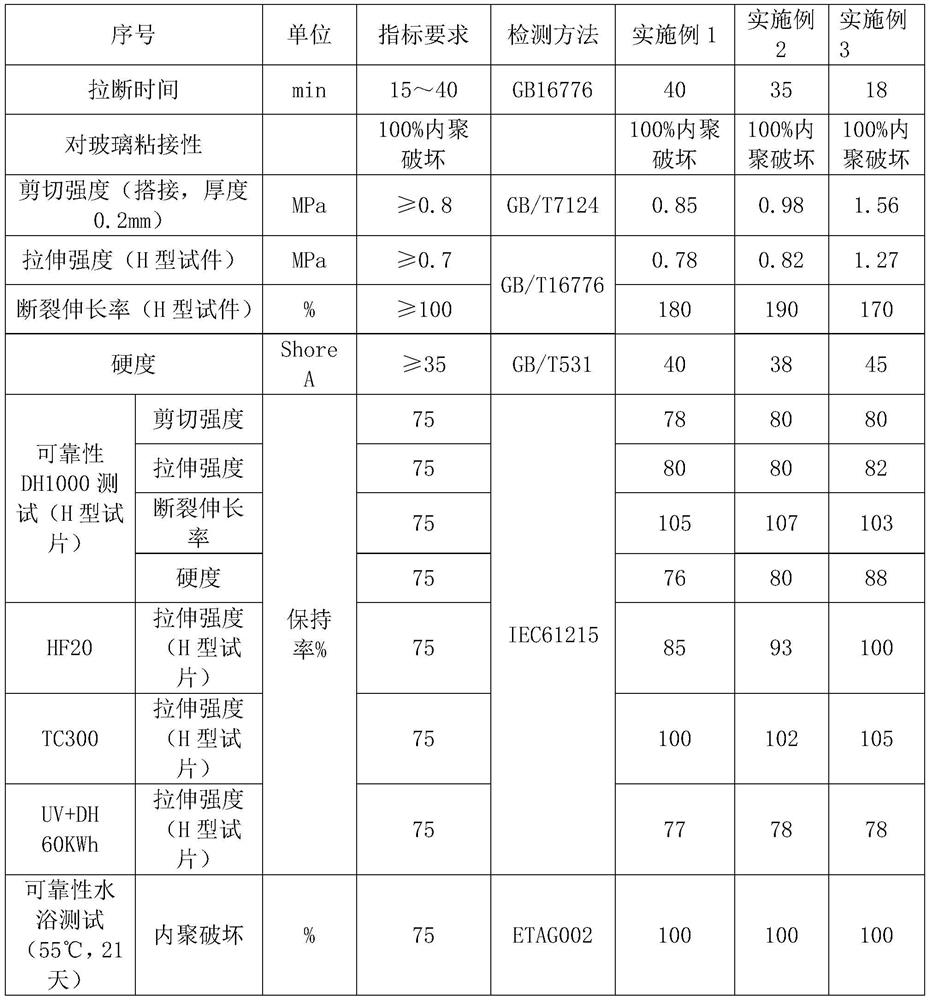
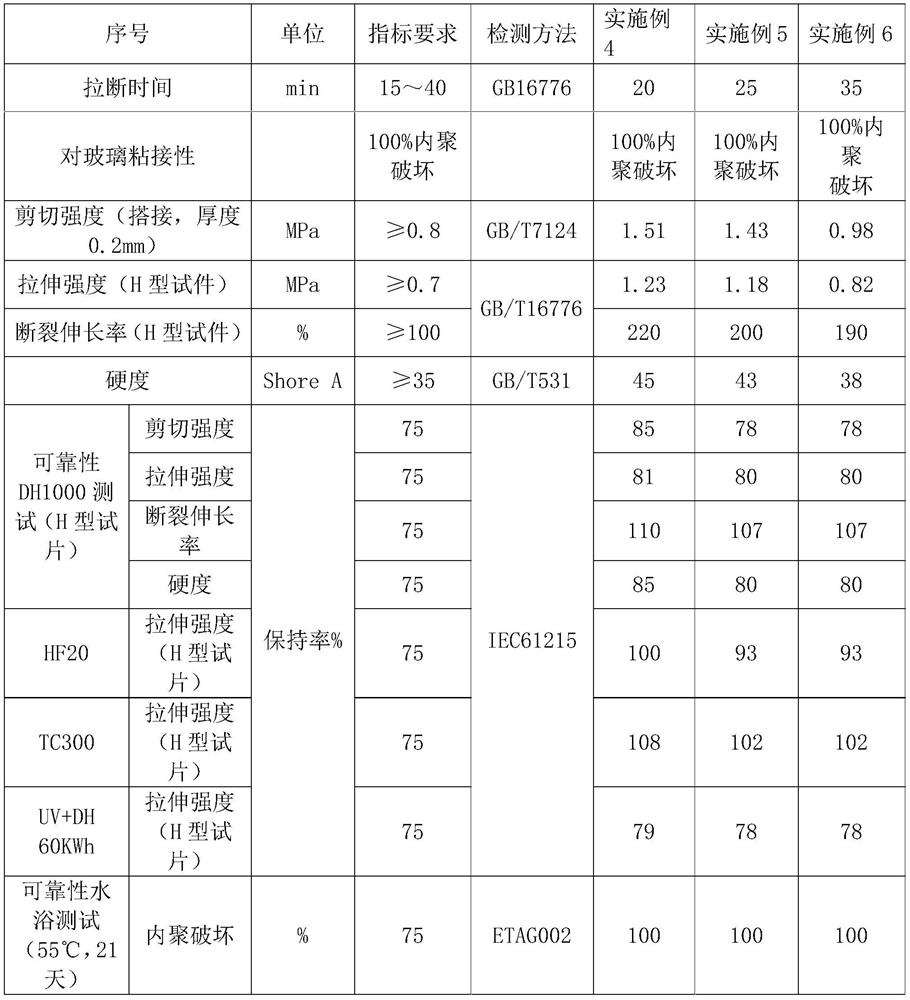
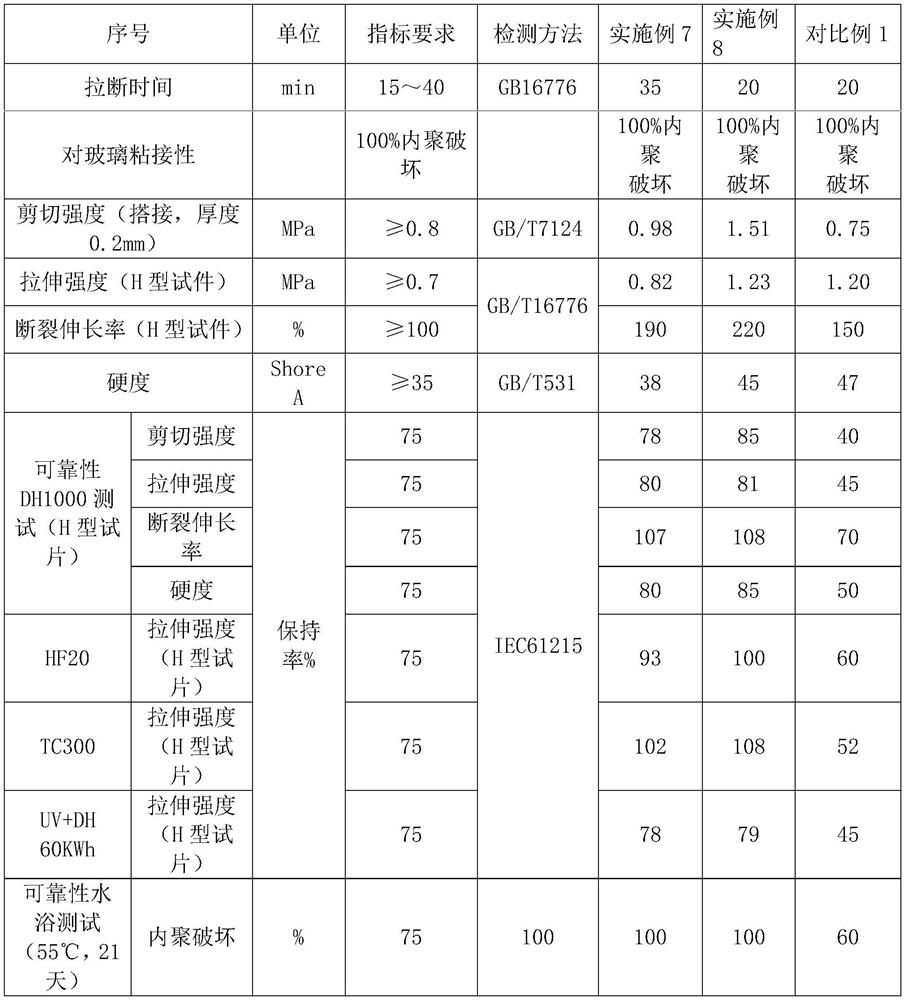
Moisture-resistant and heat-resistant high-strength silicone structural adhesive special for photovoltaic assemblies
ActiveCN107880797AExcellent heat and humidity resistanceHumidity has little effectNon-macromolecular adhesive additivesOrganic non-macromolecular adhesiveAdhesiveAntioxidant
The invention discloses a moisture-resistant and heat-resistant high-strength silicone structural adhesive special for photovoltaic assemblies. The moisture-resistant and heat-resistant high-strengthsilicone structural adhesive special for the photovoltaic assemblies consists of an A component and a B component according to a volume ratio of 10:1, wherein the A component consists of hydroxyl-terminated polydimethylsiloxane, a thixotropic agent, a reinforcing filler, a heat-resistant filler and a plasticizer; and the B component consists of methyl-terminated polydimethylsiloxane, pigment carbon black, a silane coupling agent, an active hydrogen blocking agent, a crosslinker , a catalyst, an antioxidant, a light stabilizer, and a deep curing agent. According to the moisture-resistant and heat-resistant high-strength silicone structural adhesive special for the photovoltaic assemblies, the problem that currently strength of a silicone structural adhesive decreases after damp-heat aging is solved; and the moisture-resistant and heat-resistant high-strength silicone structural adhesive special for the photovoltaic assemblies is applicable to the fields of structural bonding of double glass assembly back tracks, and between hanging buckles and glass, and bonding and sealing of photovoltaic assembly frames, and junction boxes, etc.; and the structural adhesive has high strength, excellent heat and humidity resistance and adhesion, and can play a structural adhesive role.
Owner:ZHEJIANG FORST NEW MATERIAL RES INST CO LTD
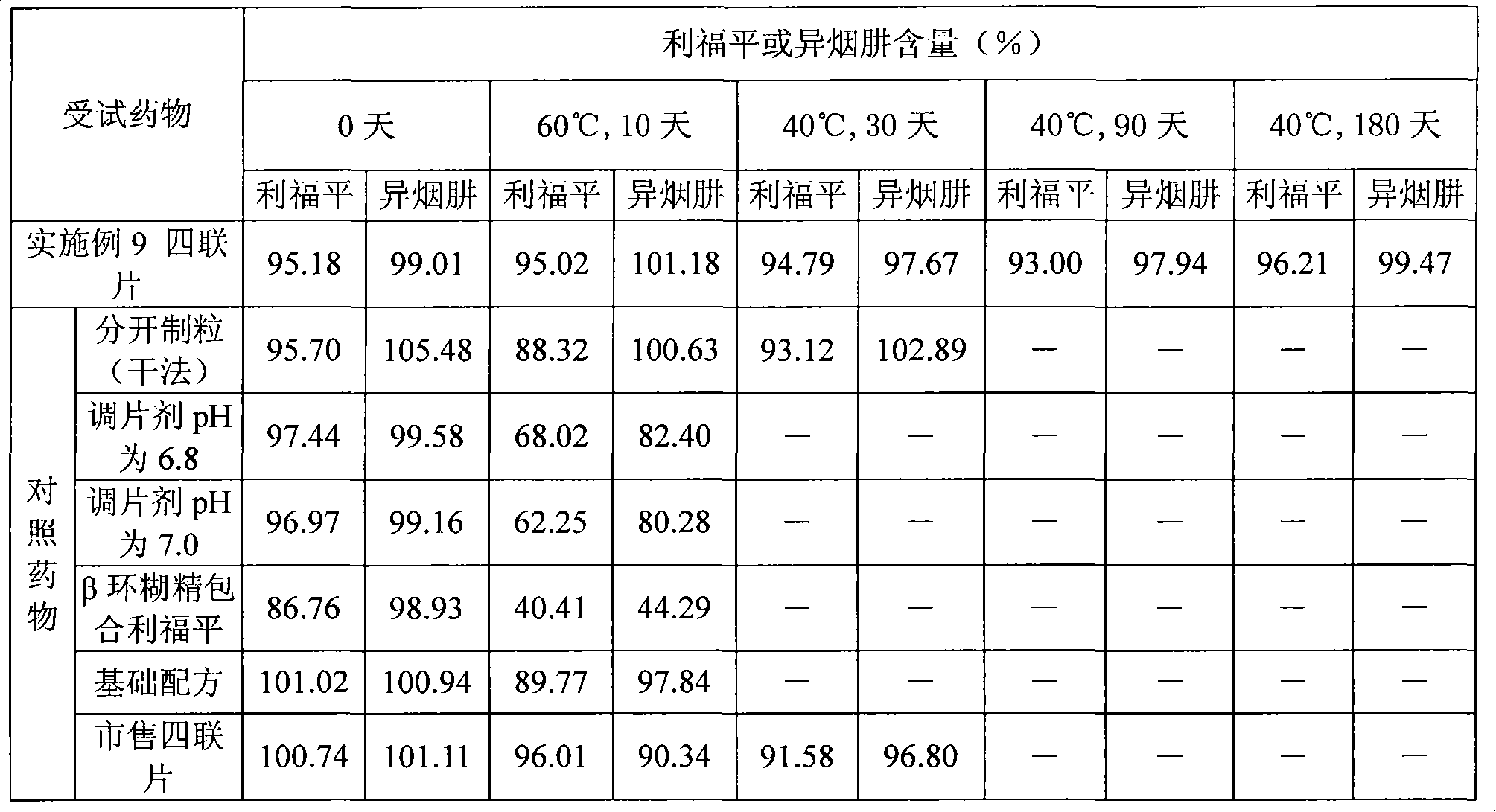
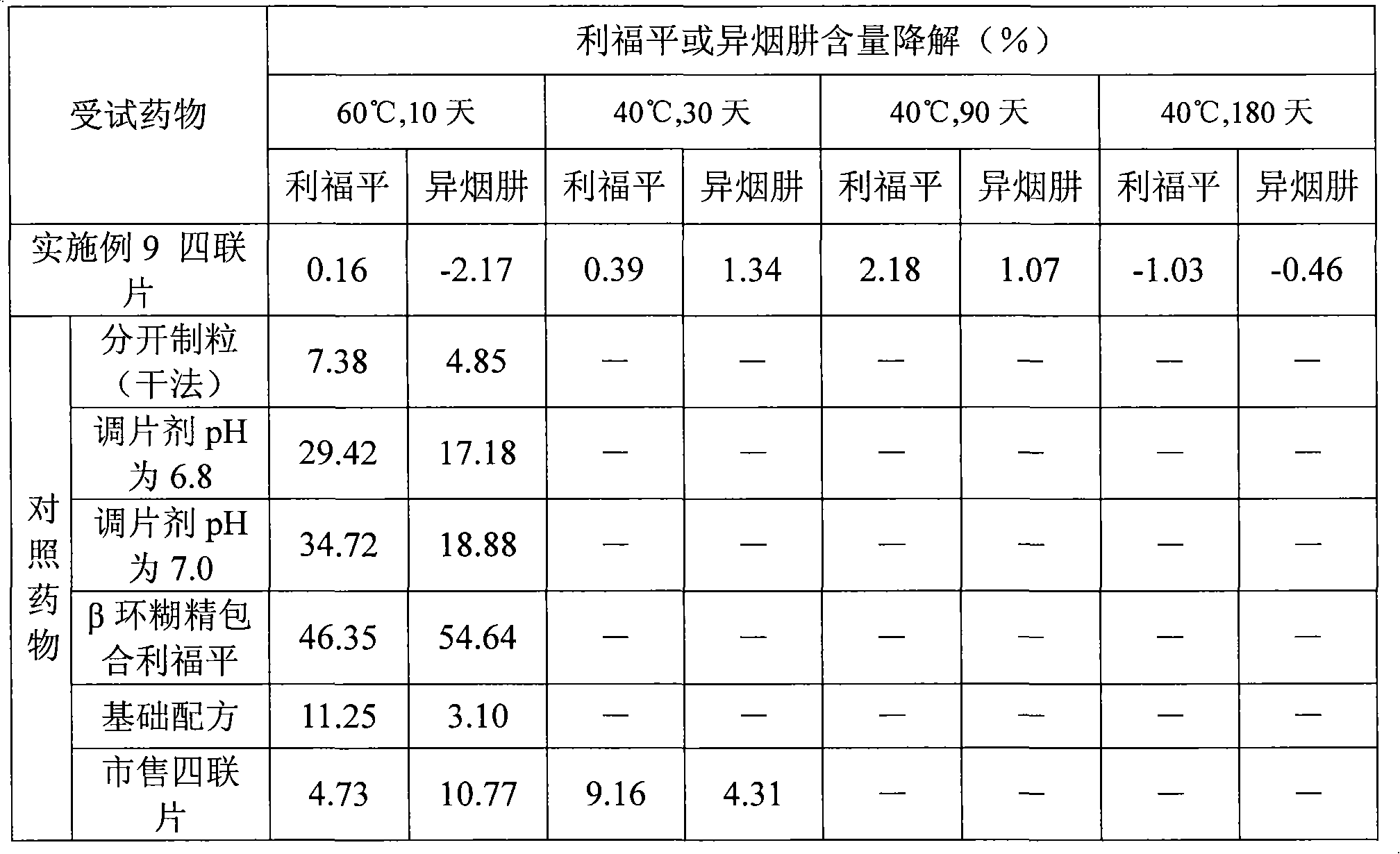
Medicine solid compound preparation and preparation method thereof
InactiveCN101601641AGood storage stabilityReduce storage requirementsOrganic active ingredientsDrageesBiomedical engineeringCompounded preparations
The invention relates to a medicine solid compound preparation which contains a medicine inner core prepared of one or more active medicines, and a medicine cover prepared by one or more active medicines, wherein the medicine cover is covered on the medicine inner core. The medicines reacted mutually in the compound preparation are separated and are positioned in different layers in the preparation so as to avoid the interaction. The invention also discloses a preparation method of the medicine solid compound preparation.
Owner:CHONGQING HUAPONT PHARMA
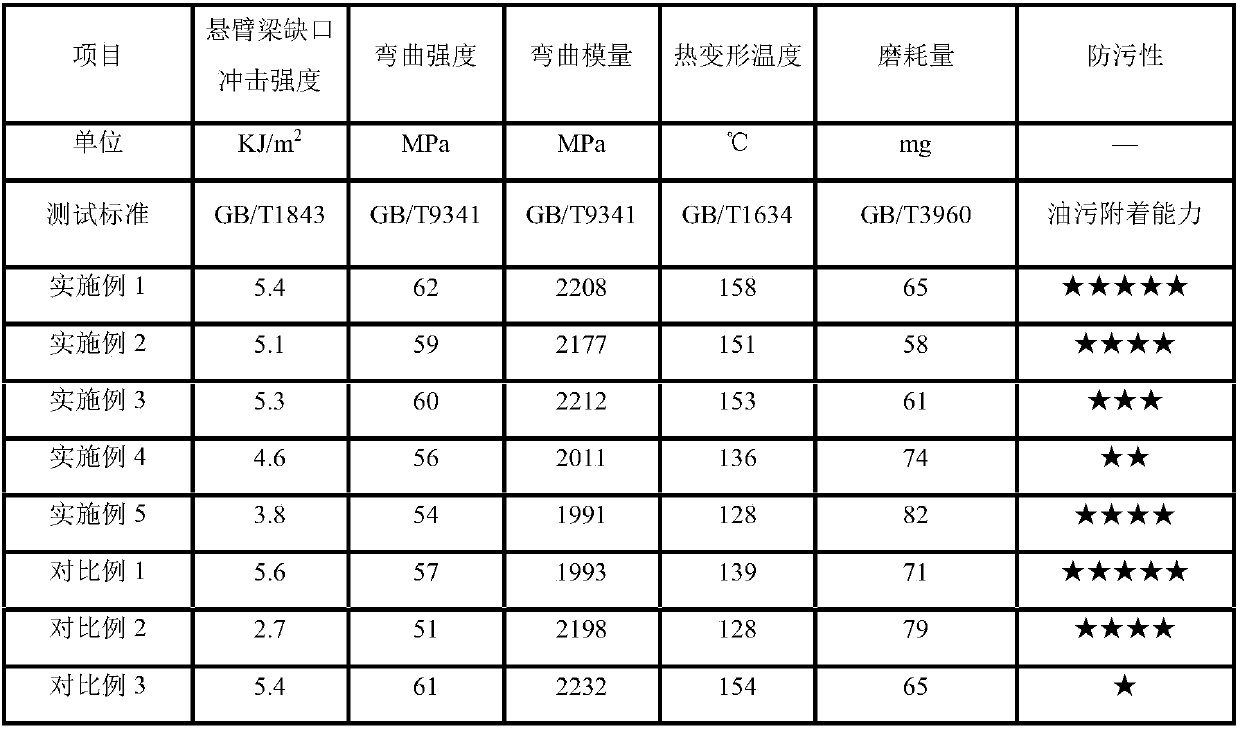
Pollution-resistance high-temperature-resistance polypropylene material and preparation method thereof
The invention belongs to the technical field of polymer composite material modification, and particularly relates to a pollution-resistance high-temperature-resistance polypropylene material and a preparation method thereof. The polypropylene material is characterized in that high-crystallization homopolymer polypropylene is taken as a basic component, polyketone resin is added to improve the wearresistance and mechanical performance of the material, barium sulfate masterbatch is taken as a filling agent to reduce dust pollution, a compatibilizer is added to improve the bonding force betweenraw materials to improve the mechanical performance of the material, organic group modified ultrahigh molecular weight polyorganosiloxane is taken as an antifouling agent to improve the water and oilresistance and the wear resistance of the material, and the anti-ageing performance of the material is improved by adopting an antioxidant. The material has the advantages of easy processing, high glossiness, high wearing resistance, pollution resistance, easy dyeing, excellent physical mechanical performance and the like, the demands by household electrical appliance products and kitchen electrical appliances on housing materials thereof in severe environments can be completely met, and the material can be widely applied to the production of plastic spare parts which need to slide or rotate in the fields of machinery, building materials, sports and the like.
Owner:GUANGZHOU SUPER DRAGON ENG PLASTICS
Methylergometrine maleate injection and preparation method thereof
ActiveCN105125481AImprove stabilityGood reproducibilityOrganic chemistryPharmaceutical delivery mechanismMethylergometrine maleateMedical prescription
The invention relates to a methylergometrine maleate injection and a preparation method thereof and belongs to the technical field of pharmaceutical preparations. The methylergometrine maleate injection comprises isoosmotic adjusting agent, buffer agent, antioxygen, metal ion complexing agent, analgesic and the like. Due to high stability of methylergometrine maleate and prescription ratio which is selected precisely and verified repeatedly, the methylergometrine maleate injection has more remarkable stability. The preparation method of the methylergometrine maleate injection is easy, in other words, the methylergometrine maleate injection can be produced by a conventional preparation method without any special equipment. Therefore advantages such as industrialization feasibility, high production efficiency, sterility guarantee, low adverse effect, controllable quality and excellent stability are achieved.
Owner:HEBEI ZHITONG BIOLOGICAL PHARMA
Structural silicone adhesive with low modulus and high volume resistivity
ActiveCN108047968ALow modulusLow Modulus High Volume ResistivityNon-macromolecular adhesive additivesMacromolecular adhesive additivesCross-linkPolymer science
The invention discloses a structural silicone adhesive with low modulus and high volume resistivity. The structural silicone adhesive is composed of a component A and a component B according to a volume ratio of 10: 1, wherein the component A is composed of hydroxyl-terminated polymethylsiloxane, a plasticizer and a reinforcing filling material; and the component B is composed of methyl-terminatedpolydimethylsiloxane, pigment carbon black, a silane coupling agent, a chain extender, an active hydrogen sealing agent, a cross-linking agent, a catalyst, an antioxidant, a light stabilizer and a deep curing agent. The structural silicone adhesive with low modulus and high volume resistivity provided by the invention improves the volume resistivity of a conventional structural adhesive from 1015omega.cm to 1016 omega.cm, increases an order of magnitude in the volume resistivity, is applicable to structural assembly of a system with photovoltaic voltage classes of 1500 V, 2000 V and even 3000 V, specifically to structural adhesion assembly of places with high requirements on insulation, such as thin film assemblies, and exerts an effect of structural adhesion.
Owner:HANGZHOU FIRST APPLIED MATERIAL CO LTD
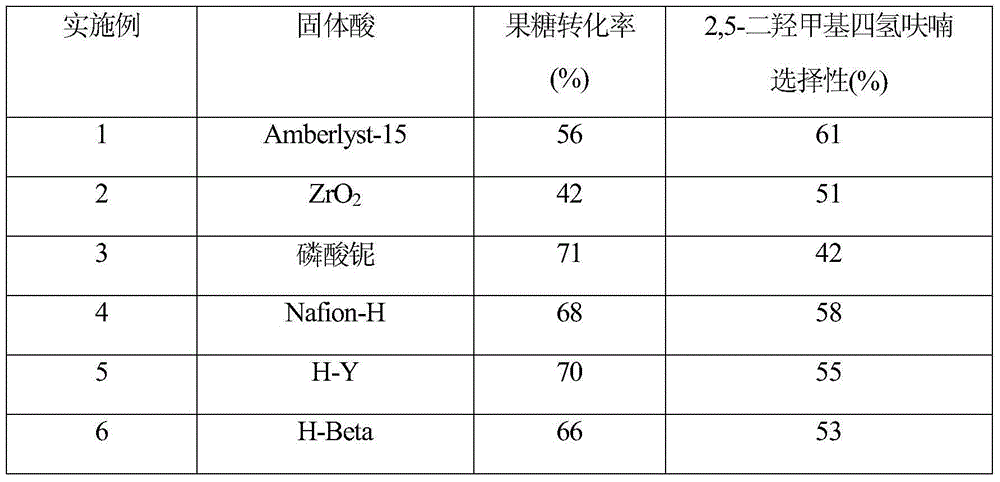
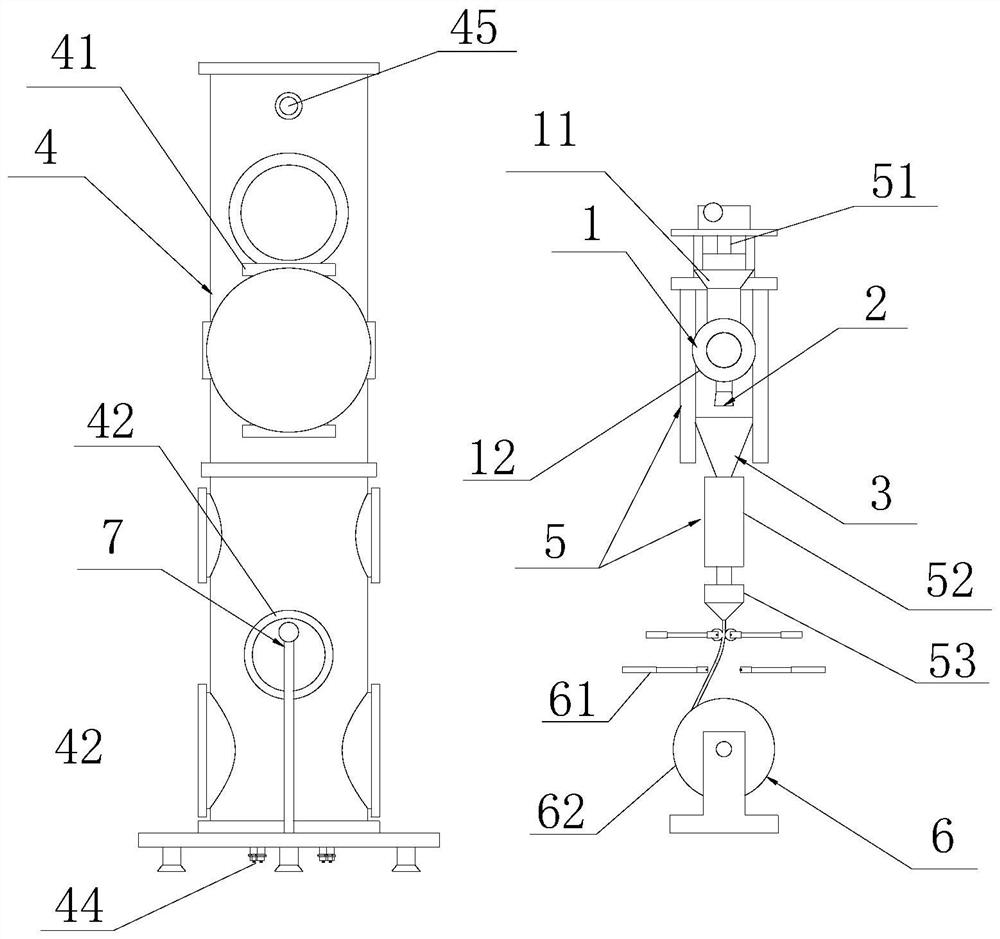
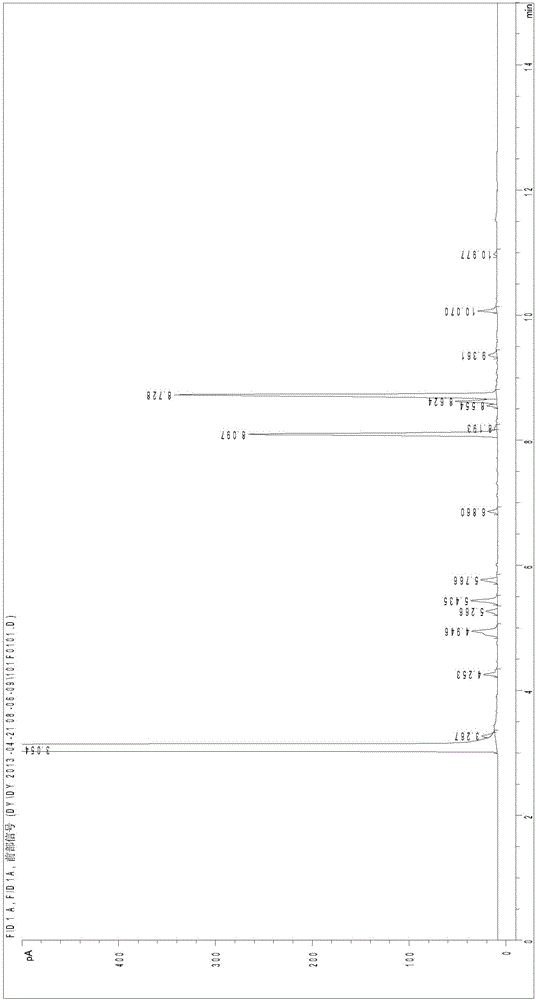
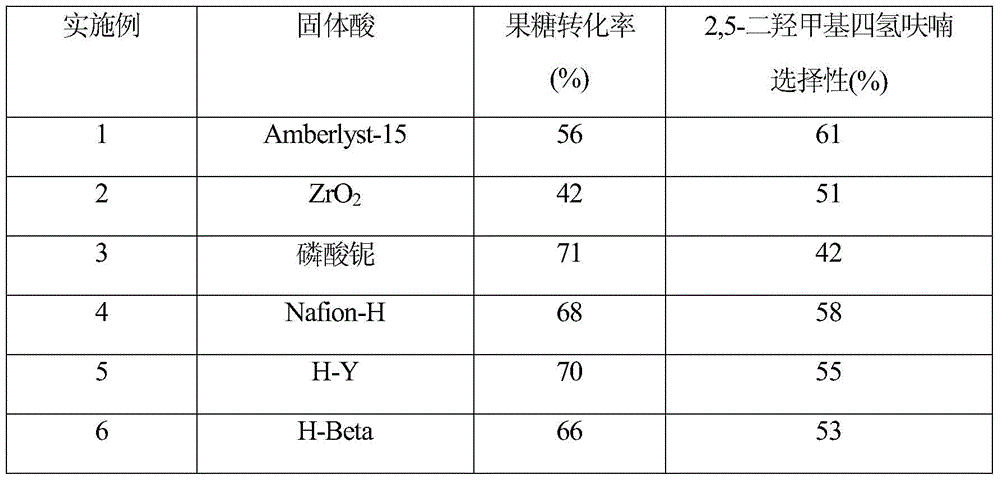
Method for directly preparing 2,5-dihydroxymethyl tetrahydrofuran from fructose
ActiveCN104672186AReduce concentrationInhibition of reduction reactionOrganic chemistryFructoseTetrahydrofuran
The invention relates to a method for directly preparing 2,5-dihydroxymethyl tetrahydrofuran from fructose, which comprises the following step: in a water / oil two-phase system, directly converting fructose into the 2,5-dihydroxymethyl tetrahydrofuran by one step under the conditions of 0.5-10 MPa H2 and 80-180 DEG C under the action of a catalyst for 0.5-16 hours. The maximum selectivity of the 2,5-dihydroxymethyl tetrahydrofuran is 72%, and the maximum yield is 56%.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Thymalfasin chitosan / beta-cyclodextrin composite microsphere drug delivery system and preparation method
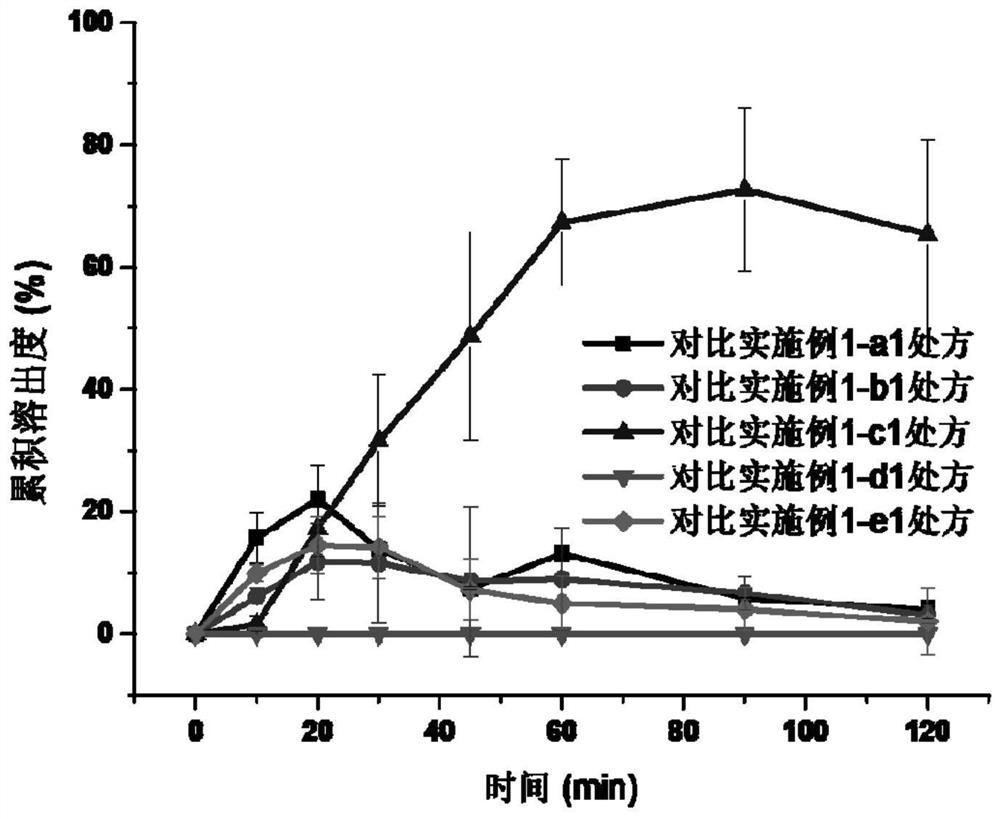
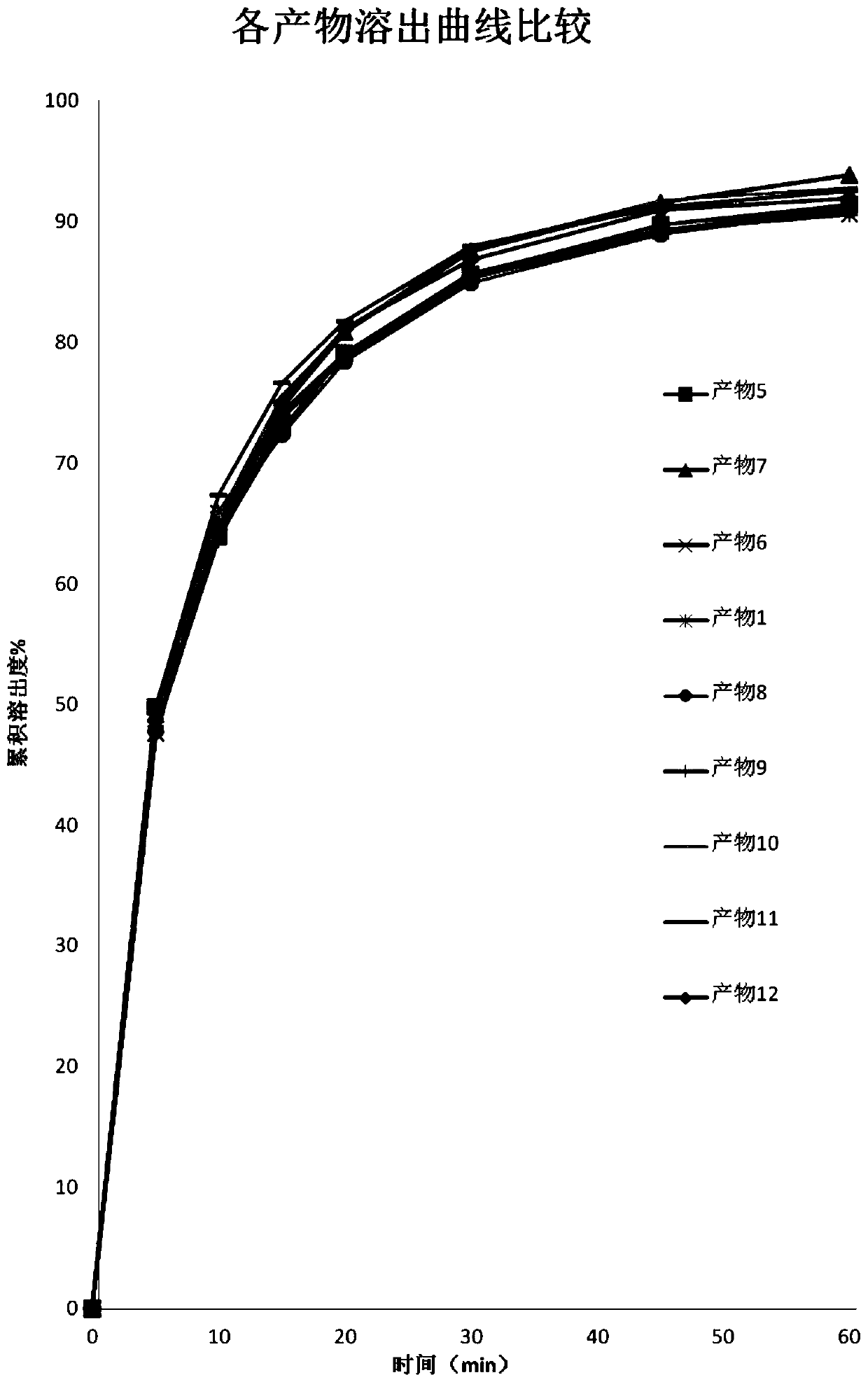
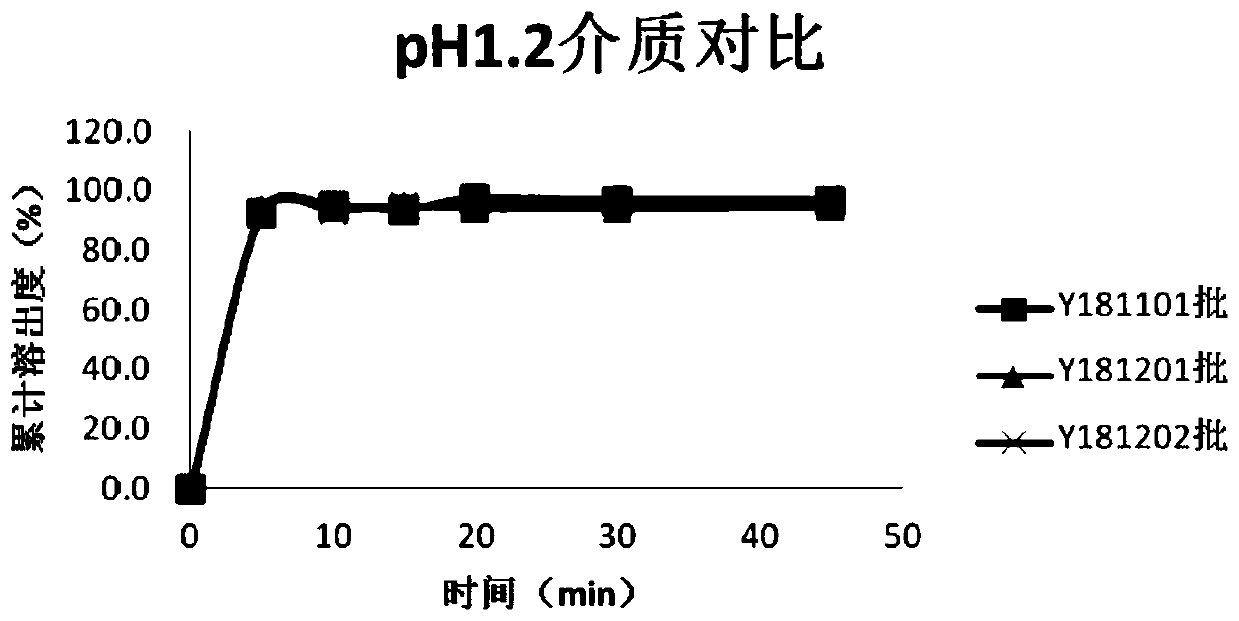
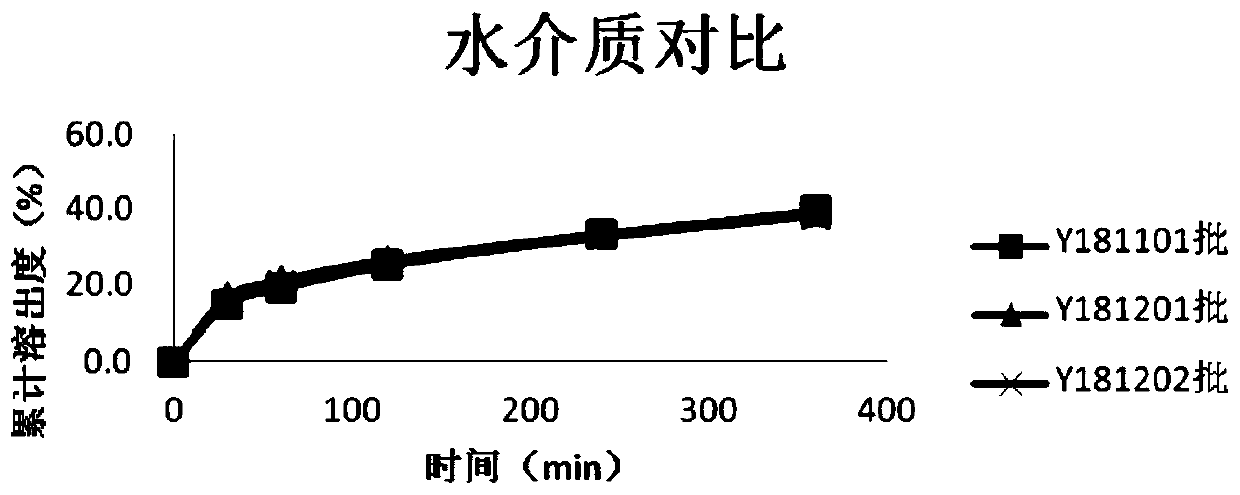
ActiveCN103126999AAvoid degradationAvoid degradation reactionsPeptide/protein ingredientsAntiviralsOral medicationMicrosphere
The invention relates to a novel thymalfasin chitosan / beta-cyclodextrin composite microsphere drug delivery system and a preparation method. Chitosan serves as a membrane material, beta-cyclodextrin serves as a clathration material, and a composite microsphere is prepared in a reversed phase suspension-chemical crosslinking method. According to the novel thymalfasin chitosan / beta-cyclodextrin composite microsphere drug delivery system, the composite microsphere carries thymalfasin. According to the novel thymalfasin drug delivery system, the obtained composite microsphere performs good protection and controlled-release functions on the thymalfasin, provides feasibility for oral administration medicines and has good economic benefits and application prospect.
High-temperature-resistant anti-aging silicone tube
The invention discloses a high-temperature-resistant anti-aging silicone tube which is prepared by squeezing a silicone rubber composite material. The silicone rubber composite material is prepared from methyl vinyl silicone rubber, ethene-acrylic rubber, ethylene-propylene-diene monomer, butadiene rubber, ethene-octylene copolymer, zinc oxide, stearic acid, 2, 5-dimethyl-2, 5-di(tert-butyl-peroxyl) hexane, methyl-tri-(butanone-oxime) silane, triallyl isocyanurate, fumed silica, nano calcium carbonate, attapulgite, lanthanum stearate, cerium hydroxide, hydroxy terminated polydimethylsiloxane, a silane coupling agent, a catalyst and an anti-aging agent. The high-temperature-resistant anti-aging silicone tube is good in high temperature resistance, excellent in anti-aging performance and long in service life.
Owner:安徽都邦电器有限公司
Pharmaceutical composition for treating steatohepatitis and preparation method of pharmaceutical composition
ActiveCN113274368AHigh dissolution rateAchieve solubilizationPowder deliveryOrganic active ingredientsChemical compoundPolyethylene glycol
The invention provides a pharmaceutical composition for treating steatohepatitis and a preparation method of the pharmaceutical composition. The pharmaceutical composition comprises the following components in parts by weight: (a) 1 part of a compound shown in a formula (I); (b) 16-600 parts of a meltable dispersion carrier, wherein the meltable dispersion carrier comprises poloxamer and polyethylene glycol, and the weight ratio of the poloxamer to the polyethylene glycol is 1:(0.5-27); and (c) 0.2-100 parts of non-volatile weak acid. The pharmaceutical composition is beneficial to greatly improving the in-vitro dissolution rate of the compound shown in the formula (I), and meanwhile, the stability of the compound shown in the formula (I) can be ensured due to mild preparation conditions.
Owner:GANNEX PHARM CO LTD
Azithromycin medicine composition and preparation method thereof
ActiveCN110755404AAdjust the dissolution rateDoes not change the degree of dissolutionAntibacterial agentsOrganic active ingredientsAzithromycinEfficacy
The invention provides an azithromycin medicine composition and a preparation method thereof. The azithromycin medicine composition comprises medicine granules containing azithromycin, an isolation layer, a taste masking layer and a taste modifying layer, wherein the isolation layer, the taste masking layer and the taste modifying layer sequentially coat the medicine granules in a form of coating.The isolation layer, the taste masking layer and the taste modifying layer of the azithromycin medicine composition are prepared into coats to coat sequentially coat the medicine granules in the formof coating, so that a medicine component release curve is more gentle, and the occurrence of adverse reactions of gastrointestinal tracts can be reduced effectively on the premise that the efficacy and bioavailability are guaranteed. Meanwhile, the azithromycin medicine composition reduces the dosage of a taste modifying agent, is safer to take by children and keeps a good taste.
Owner:长春雷允上药业有限公司
Synthetic method of azithromycin rearrangement impurity R
ActiveCN108530494AGood qualityEasy to quantitative controlSugar derivativesSugar derivatives preparationPyranoseAzithromycin
The invention belongs to the technical field of chemistry, and particularly relates to a synthetic method of an azithromycin rearrangement impurity R. The method mainly comprises the steps that (3R,4R,5S,6R,9R,10S,11S,12R,13R,15R,Z)-12-[[3,4,6-trideoxy-3-(dimethylamino)-beta-D-wood-pyranose group]oxygen]-6-ethyl-4,5-dihydroxyl-10-[(2,6-dideoxy-3-C-methyl-a-L-nuclear-pyranose group)oxygen]-3,5,9,11,13,15-hexamethyl-7,16-dioxy-2-azabicyclo[11.2.1]cetane-1-alkene-8-ketone(compound III) is subjected to acidification rearrangement, and after refining is conducted, the rearrangement impurity R withthe purity being 99.5% or above is obtained. The synthesized high-purity azithromycin rearrangement impurity R serves as an impurity standard substance of finished production detection, locating and qualification on the impurity are enhanced conveniently, and quality control over azithromycin active pharmaceutical ingredient is improved.
Owner:HEC PHARM
Application of binaphthol derivatives in aspect of active free radical photopolymerization reaction
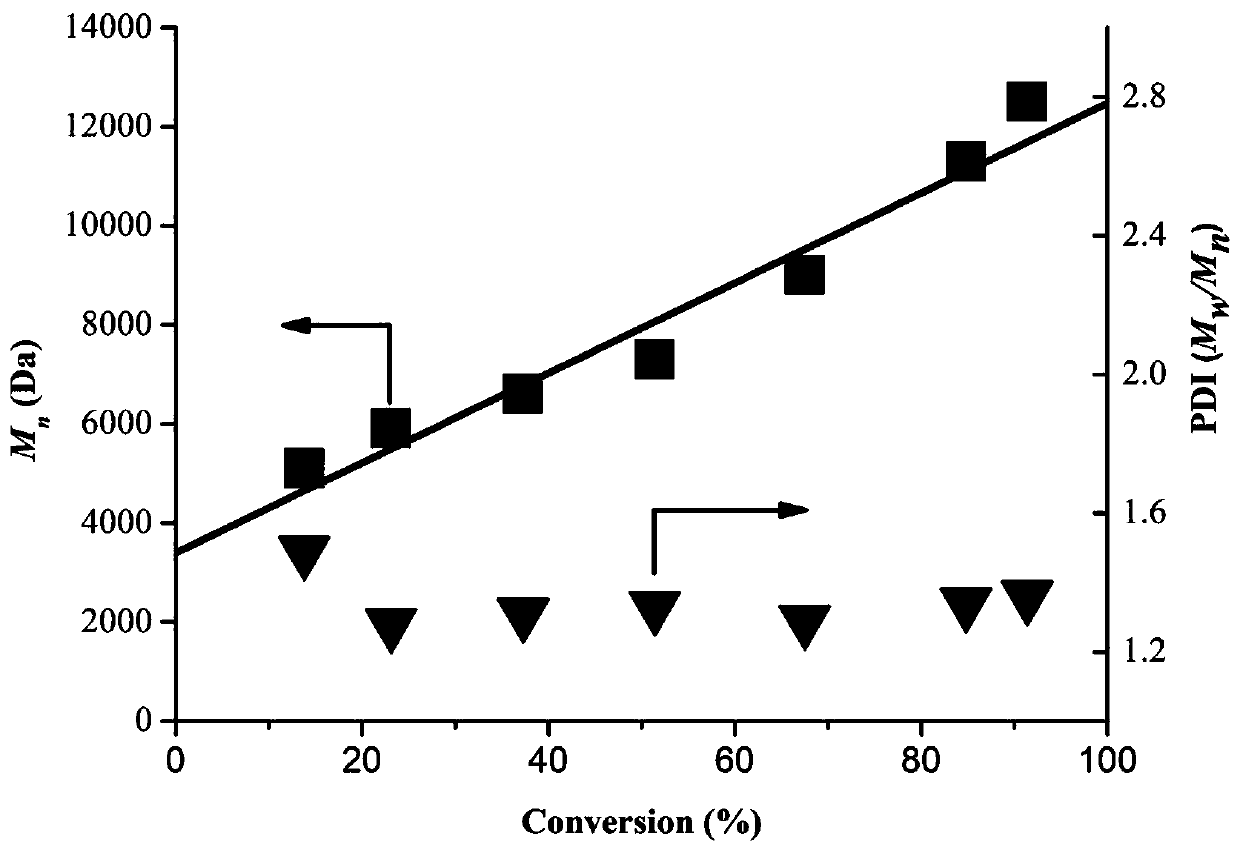
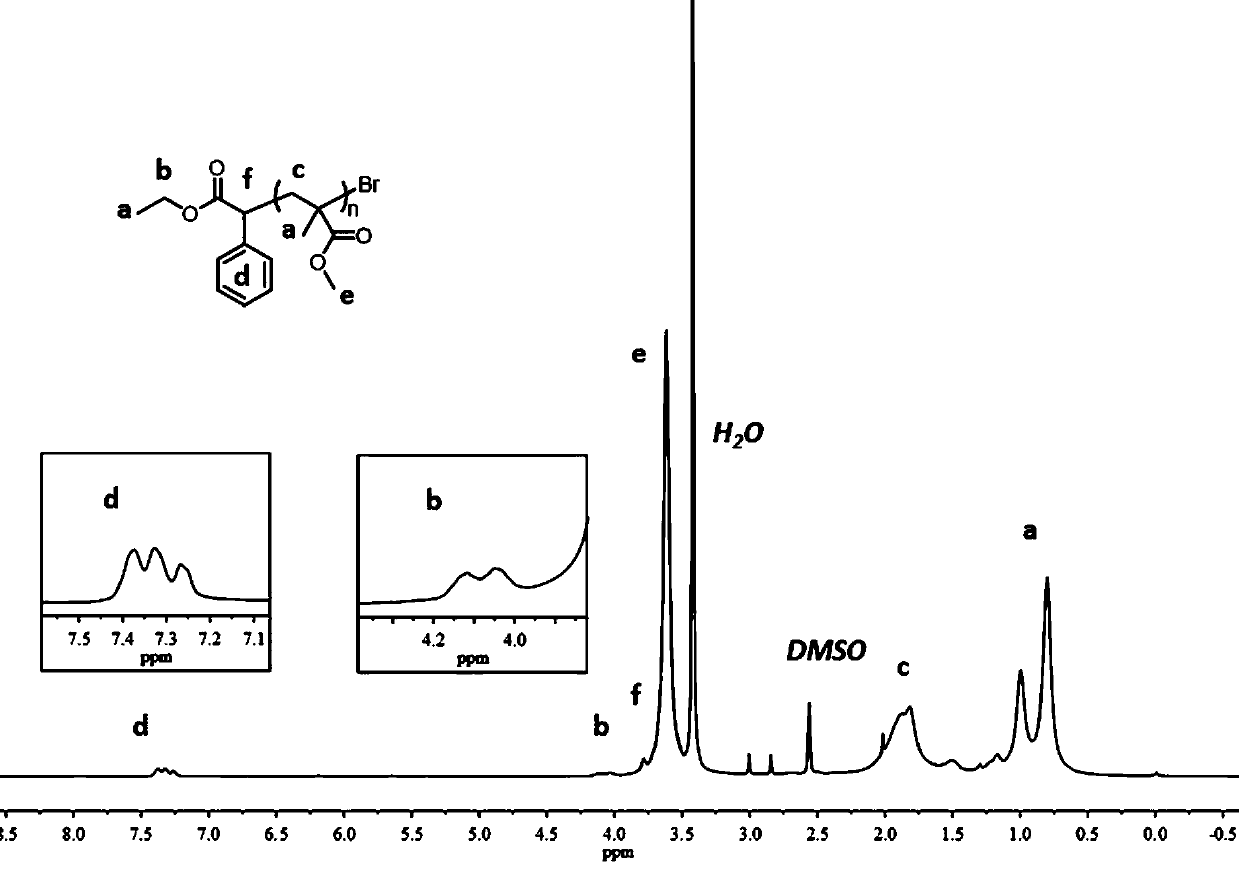
The invention discloses application of binaphthol derivatives in the aspect of active free radical photopolymerization reaction, and relates to the field of controllable active free radical polymerization catalyzed by organic photocatalysts, in particular to an organic catalytic system which takes 1, 1'-bi-2, 2'-naphthol derivatives as organic photocatalysts and generates activity-controllable free radical photopolymerization under visible light. According to the invention, a BINOL polymer is used as an organic photooxidation reduction catalyst, so that rapid reversible equilibrium between dormant species and an active chain can be achieved, the polymerization reaction has controllability, and a novel application method for the BINOL organic polymer in the organic photocatalytic active free radical polymerization is provided while a homopolymer and a block copolymer with characteristics of the controllable molecular weight and the low polydispersity can be prepared; and moreover, the organic catalytic system provided by the invention has the characteristics of high efficiency, low cost and easiness in operation, and the prepared polymer has the characteristics of the controllable molecular weight and the narrow polydispersity, and conforms to the production concept of environment-friendly green production.
Owner:FUZHOU UNIV
Anti-aging PVC (Polyvinyl Chloride) board with excellent thermal stability and preparation method of anti-aging PVC board
The invention discloses an anti-aging PVC (Polyvinyl Chloride) board with excellent thermal stability and a preparation method of the anti-aging PVC board. The anti-aging PVC board comprises the following raw materials in parts by mass: chloroethylene, epoxy compounds, a rare earth catalyst, di-cetyl peroxydicarbonate, modified montmorillonite, nano calcium carbonate, 2-(2-hydroxy-4-hexoxyphenyl)-4,6-diphenyl-1,3,5-triazine, 2,2'-methylene-bis(4-tert-butyl-6-benzotriazolyl) phenol, pentaerythritol ester, a dispersing agent and a plasticizer. The PVC board prepared by the preparation method disclosed by the invention is excellent in thermal stability and is difficult to decompose and discolor under light and high temperature conditions, release of toxic gases is reduced, and the service life is prolonged.
Owner:安徽优丽普科技有限公司
Emulsion storage bottle capable of keeping emulsion stable
InactiveCN112173360AExtended service lifeAvoid corrosionBottlesSupporting meansBottle capThreaded rod
The invention discloses an emulsion storage bottle capable of keeping emulsion stable. The emulsion storage bottle comprises a bottle body, an emulsion pressing unit, a mounting unit and a bottle cap.According to the bottle body, the bottom end of the bottle cap is matched with the top end of the bottle body, fixing grooves are formed in both the left side and the right side of the interior of the top end of the bottle body, the mounting unit is arranged at the top end of the bottle cap, a protection unit is arranged at the bottom end of the bottle body, and a light shielding unit is arrangedat the front end of the outer side face of the bottle body. The emulsion pressing unit comprises a filtering connecting piece, a feeding pipe, a push plate, a threaded rod, a rotating wheel and a discharging pipe. The rotating wheel is arranged at the bottom end of the bottle body, the top end of the rotating wheel is connected with the bottom end of the threaded rod, the threaded rod is locatedat the bottom end of the interior of the bottle body and is in threaded connection with a threaded hole in the middle of the push plate, and a filtering connecting piece is arranged at the top end ofthe interior of the bottle cap. A feeding pipe is arranged at the bottom end of the filtering connecting piece. The emulsion storage bottle capable of keeping the emulsion stable is easy to use, convenient to replace, high in practicability and good in protection effect.
Owner:温州飞创化妆品有限公司
Method for synthesizing azithromycin rearrangement impurity lactam
ActiveCN109575092AGood qualityEasy to quantitative controlSugar derivativesSugar derivatives preparationAzithromycinSynthesis methods
The invention belongs to the technical field of chemistry, and particularly relates to a synthesis method for azithromycin rearrangement impurity lactam. The synthesis method comprises the main step that (3R,4R,5S,6R,9R,10S,11S,12R,13R,15R,Z)-12-[[3,4,6-trideoxy-3-(dimethylamino)-beta-D-xylo-hexopyranosyl]oxy]-6-ethyl-4,5-dihydroxy-10-[(2,6-dideoxy-3-C-methyl-3-O-methyl-a-L-ribo-hexopyranosyl)oxy]-3,5,9,11,13,15-hexamethyl-7,16-dioxa-2-azabicyclo[11.2.1]hexadec-1-en-8-one (a compound II ) is subjected to acidification and alkalization rearrangement and then is refined so as to obtain the rearrangement impurity lactam with the purity of 99.5% or above. The synthesized high-purity azithromycin rearrangement impurity lactam serves as an impurity standard substance for finished product detection, so that positioning and nature determining on impurities is facilitated, and the quality control over azithromycin crude drugs is improved.
Owner:HEC PHARM
A kind of azithromycin pharmaceutical composition and preparation method thereof
ActiveCN110755404BAdjust the dissolution rateDoes not change the degree of dissolutionAntibacterial agentsOrganic active ingredientsEfficacyPharmaceutical Substances
The invention provides a pharmaceutical composition of azithromycin and a preparation method thereof, which comprises drug granules containing azithromycin, an isolation layer, a taste-masking layer and a flavor-correcting layer, wherein the isolation layer, the taste-masking layer and the flavor-correcting layer are sequentially coated with The drug particles are coated in the form of a coating. The azithromycin pharmaceutical composition forms a coating in which the isolation layer, the taste-masking layer and the flavor-correcting layer are successively coated on the azithromycin drug granules, so that the release curve of the drug components is relatively gentle, and the drug effect and bioavailability can be ensured. Under the premise of effectively reducing the incidence of gastrointestinal adverse reactions. At the same time, the azithromycin pharmaceutical composition of the present invention reduces the dosage of flavoring agents, making it safer for children to take, while maintaining a good taste.
Owner:长春雷允上药业有限公司
A kind of synthesis method of azithromycin rearrangement impurity lactam
ActiveCN109575092BGood qualityEasy to quantitative controlSugar derivativesSugar derivatives preparationHexadecanePyranose
Owner:HEC PHARM CO LTD
A low modulus high volume resistivity silicone structural adhesive
ActiveCN108047968BLow modulusLow Modulus High Volume ResistivityNon-macromolecular adhesive additivesMacromolecular adhesive additivesPolymer sciencePtru catalyst
The invention discloses a structural silicone adhesive with low modulus and high volume resistivity. The structural silicone adhesive is composed of a component A and a component B according to a volume ratio of 10: 1, wherein the component A is composed of hydroxyl-terminated polymethylsiloxane, a plasticizer and a reinforcing filling material; and the component B is composed of methyl-terminatedpolydimethylsiloxane, pigment carbon black, a silane coupling agent, a chain extender, an active hydrogen sealing agent, a cross-linking agent, a catalyst, an antioxidant, a light stabilizer and a deep curing agent. The structural silicone adhesive with low modulus and high volume resistivity provided by the invention improves the volume resistivity of a conventional structural adhesive from 1015omega.cm to 1016 omega.cm, increases an order of magnitude in the volume resistivity, is applicable to structural assembly of a system with photovoltaic voltage classes of 1500 V, 2000 V and even 3000 V, specifically to structural adhesion assembly of places with high requirements on insulation, such as thin film assemblies, and exerts an effect of structural adhesion.
Owner:HANGZHOU FIRST APPLIED MATERIAL CO LTD
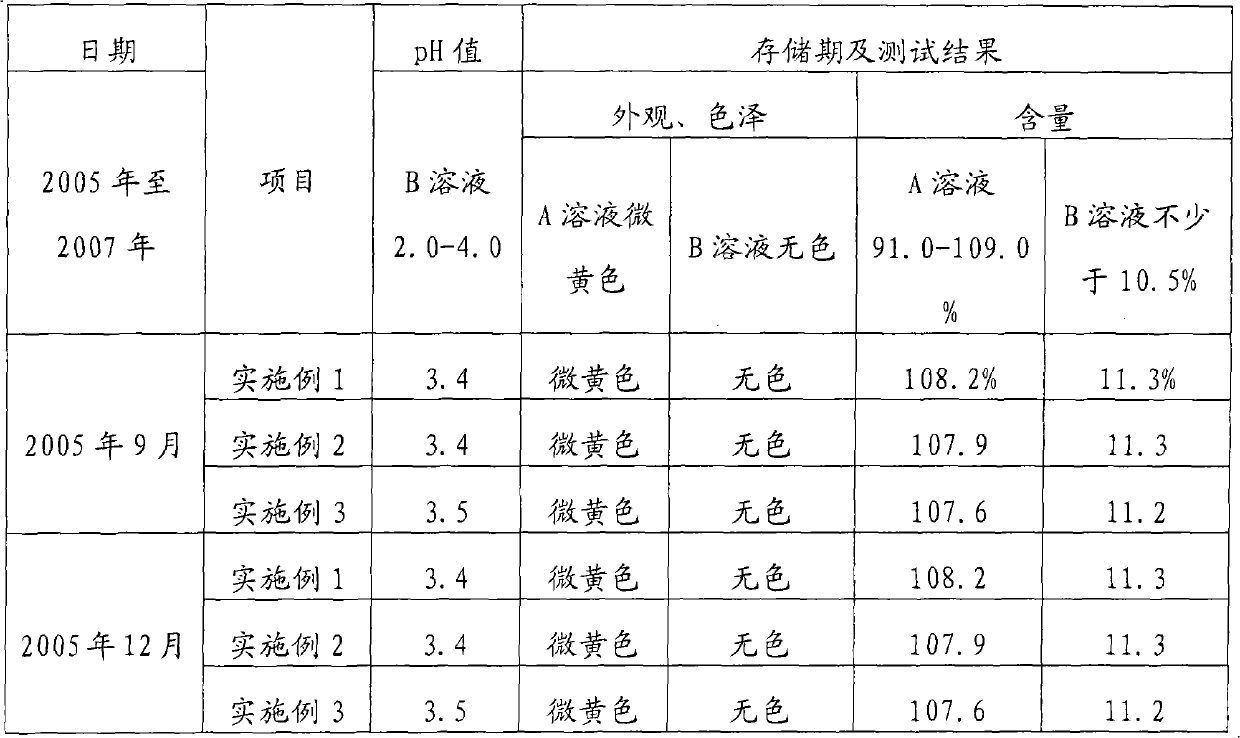
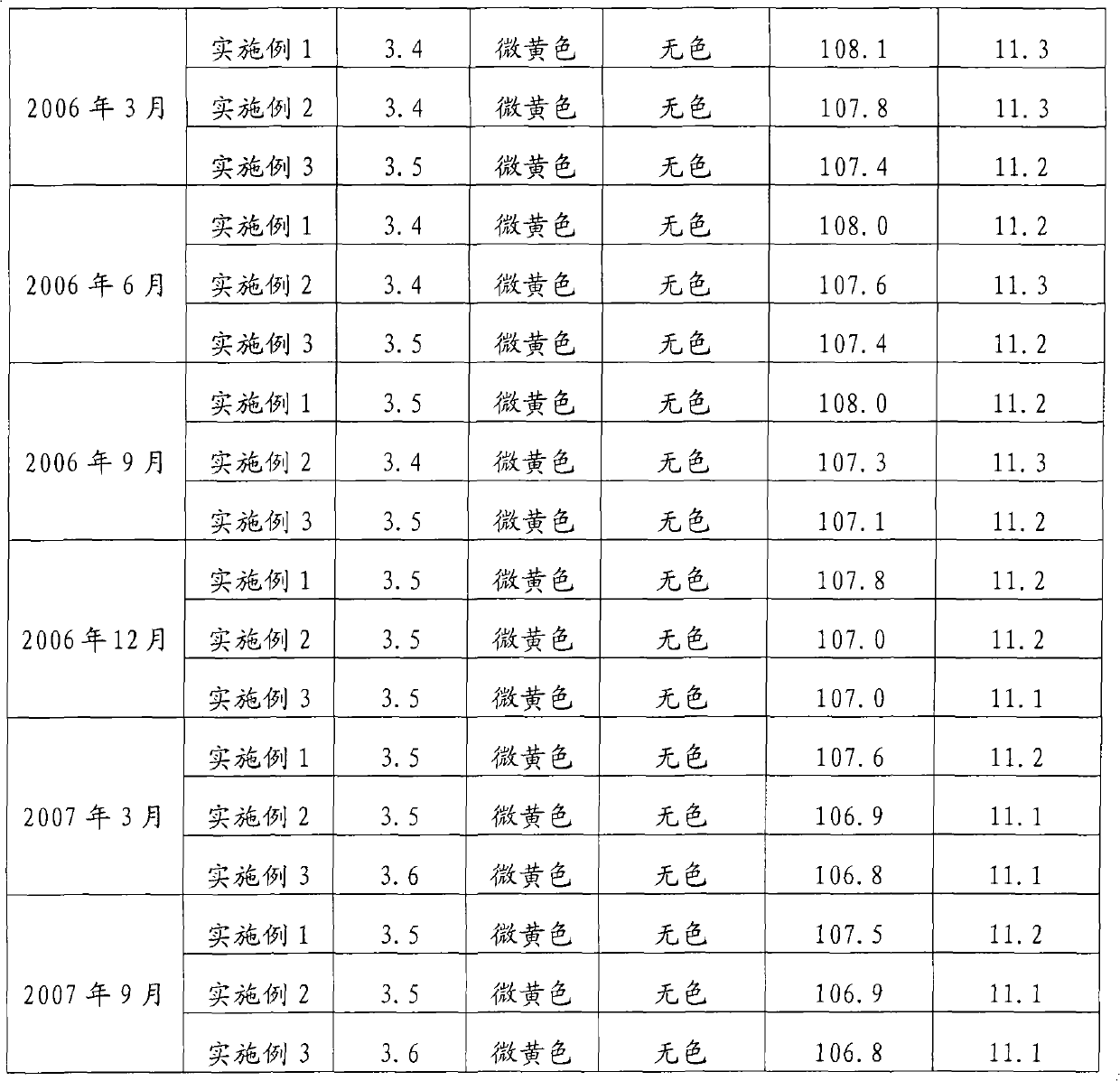
Disinfectant
ActiveCN101878779BAvoid degradation reactionsImprove performanceBiocideDisinfectantsOxalateCacodylic acid
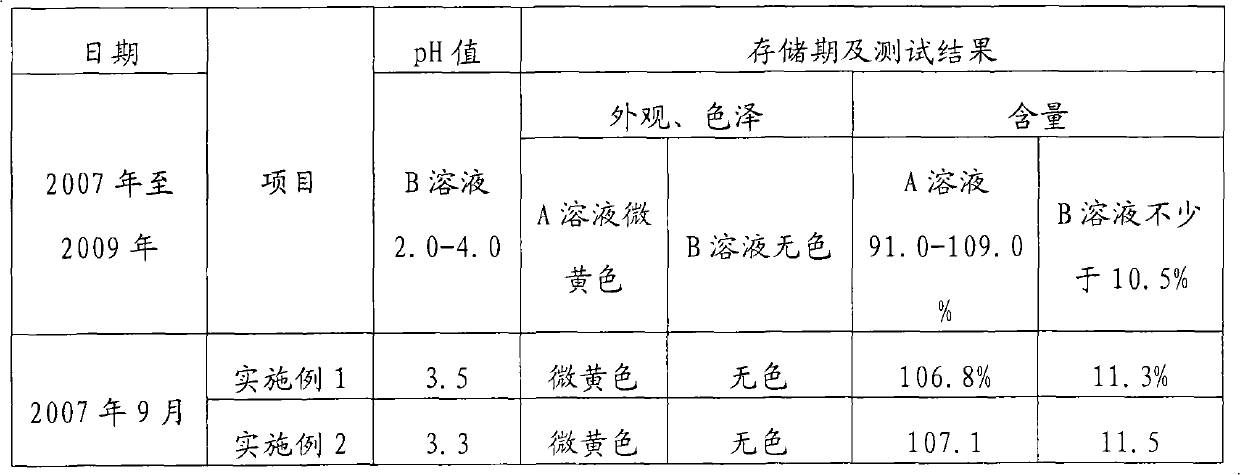
The invention discloses a disinfectant. The disinfectant is solution prepared by mixing A solution and B solution, wherein the A solution is prepared by mixing 15 to 20 mass percent solution of sodium chlorite and the solution of stabilizer, while the B solution is the mixed aqueous solution of citric acid and oxalic acid. The solution of stabilizer is 5 to 10 mass percent solution uniformly prepared from dianine sulfate, triethanolamine, ammonium persulfate and alchlor, and the mass ratio of the dianine sulfate to the triethanolamine to the ammonium persulfate to the alchlor is 1: 0.5-1.5: 0.5-1: 1.5-2.5. The volume of the solution of stabilizer is 2 to 5 percent based on the total volume of the A solution. The mass ratio of the citric acid to the oxalic acid is 1: 2-4, and the content of the B solution is 40 to 50 mass percent. The used volume ratio of the A solution to the B solution is 1-4: 1. The disinfectant has the advantages that: the medicinal performance is stable; disinfectant damages to an environment and human bodies are avoided in the use and production processes of the disinfectant; and apparatus and equipment are not corroded during the production.
Owner:BEIJING KEJIA YONGHAO TECH & TRADE
A kind of methysergide maleate injection and preparation method thereof
ActiveCN105125481BImprove stabilityGood reproducibilityOrganic chemistryPharmaceutical delivery mechanismMethylergometrine maleateMedical prescription
Owner:HEBEI ZHITONG BIOLOGICAL PHARMA
Application of Binaphthol Derivatives in Living Radical Photopolymerization
ActiveCN110885388BReduce air sensitivityWell formedLiving free-radical polymerizationOrganocatalysis
Owner:FUZHOU UNIV
Thymalfasin chitosan / beta-cyclodextrin composite microsphere drug delivery system and preparation method
ActiveCN103126999BAvoid degradationAvoid degradation reactionsPeptide/protein ingredientsAntiviralsControlled releaseOral medication
The invention relates to a novel thymalfasin chitosan / beta-cyclodextrin composite microsphere drug delivery system and a preparation method. Chitosan serves as a membrane material, beta-cyclodextrin serves as a clathration material, and a composite microsphere is prepared in a reversed phase suspension-chemical crosslinking method. According to the novel thymalfasin chitosan / beta-cyclodextrin composite microsphere drug delivery system, the composite microsphere carries thymalfasin. According to the novel thymalfasin drug delivery system, the obtained composite microsphere performs good protection and controlled-release functions on the thymalfasin, provides feasibility for oral administration medicines and has good economic benefits and application prospect.
Owner:GUANGDONG XIANQIANG PHARMA
Molten resin defoaming method and spinning forming method
ActiveCN112391685ALarge specific surface areaQuality improvementSpinning solution filteringMelt spinning methodsYarnPolymer science
The invention discloses a molten resin defoaming method and a spinning forming method. The defoaming method comprises the following steps: S1, feeding; S2, in a vacuum environment, carrying out spinning treatment on molten resin; and S3, in the vacuum environment, carrying out defoaming treatment on the resin subjected to spinning. According to the molten resin defoaming method provided by the invention, the molten resin is jetted into filaments by a spinning process, the specific surface area of the resin filaments is increased, bubbles are extremely easy to escape from the surfaces or the interiors of the resin filaments, and the defoaming effect is remarkable. The invention further provides a spinning forming method, the defoamed resin is spun, the bubbles in resin yarn are remarkably reduced, and the quality of the resin yarn is remarkably improved.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Manufacturing method of ultra-barrier hollow container
The invention discloses a method for manufacturing a super-barrier multi-layer hollow container, comprising: a blow molding process, in which a preform made of resin material is injected into the preform with a first air pressure P1 Blowing nitrogen to form a hollow body with a first volume; mixing blow molding process, reducing the air pressure to the second air pressure P2, and blowing fluorine gas into the hollow body with a third air pressure P3 to form a hollow body with a second volume. Embryo body, wherein, P3 is greater than P1; cycle blow molding process, reduce the air pressure to the fourth air pressure P4, blow nitrogen into the hollow body with the fifth air pressure P5, wherein, P5 is less than P3, repeat this step until After the blowing time is over, the hollow container of a predetermined shape is formed, and the outer wall of the hollow container is at least partially attached to the cavity wall of the extrusion blow mold. The invention can make the barrier property of the produced hollow container stronger, expand its application range, and greatly improve the economic benefit while improving the production efficiency.
Owner:JRB PACKING CO LTD
A method for directly preparing 2,5-dimethyloltetrahydrofuran from fructose
ActiveCN104672186BReduce concentrationInhibition of reduction reactionOrganic chemistryFructoseTetrahydrofuran
A method for directly preparing 2,5-dimethyloltetrahydrofuran from fructose, the method is in a water / oil two-phase system, under the condition of 0.5-10MPa H2, 80-180°C, under the action of a catalyst for 0.5-16h Direct one-step conversion of fructose to 2,5‑dimethyloltetrahydrofuran. The highest selectivity of 2,5-dimethyloltetrahydrofuran is 72%, and the highest yield is 56%.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
A kind of synthetic method of azithromycin rearrangement impurity r
ActiveCN108530494BGood qualityEasy to quantitative controlSugar derivativesSugar derivatives preparationPyranoseAzabicyclane
The invention belongs to the technical field of chemistry, and particularly relates to a synthetic method of an azithromycin rearrangement impurity R. The method mainly comprises the steps that (3R,4R,5S,6R,9R,10S,11S,12R,13R,15R,Z)-12-[[3,4,6-trideoxy-3-(dimethylamino)-beta-D-wood-pyranose group]oxygen]-6-ethyl-4,5-dihydroxyl-10-[(2,6-dideoxy-3-C-methyl-a-L-nuclear-pyranose group)oxygen]-3,5,9,11,13,15-hexamethyl-7,16-dioxy-2-azabicyclo[11.2.1]cetane-1-alkene-8-ketone(compound III) is subjected to acidification rearrangement, and after refining is conducted, the rearrangement impurity R withthe purity being 99.5% or above is obtained. The synthesized high-purity azithromycin rearrangement impurity R serves as an impurity standard substance of finished production detection, locating and qualification on the impurity are enhanced conveniently, and quality control over azithromycin active pharmaceutical ingredient is improved.
Owner:HEC PHARM CO LTD
Manufacturing method for super-barrier hollow container
The invention discloses a manufacturing method for a super-barrier multilayer hollow container. The manufacturing method comprises the working procedures that one-time blow forming is conducted, specifically, a pre-forming blank made of a resin material is formed in an extrusion blow die, nitrogen is blown into the pre-forming blank under the first air pressure P1, and thus a hollow blank with thefirst volume is formed; mixing blow forming is conducted, specifically, the air pressure is decreased to the second air pressure P2, fluorine is blown into the hollow blank under the third air pressure P3, and thus a hollow blank with the second volume is formed, wherein P3 is larger than P1; and circulation blow forming is conducted, specifically, the air pressure is decreased to the fourth airpressure P4, nitrogen is blown into the hollow blank under the fifth air pressure P5, wherein P5 is smaller than P3, the working procedure is repeatedly cycled till the blow time is finished and the hollow container with the predetermined shape is formed, and at least part of the outer wall of the hollow container is attached to the die cavity wall of the extrusion blow die. The produced hollow container can be higher in barrier property, the application range of the hollow container is enlarged, and the economic benefits are greatly increased while the production efficiency is improved.
Owner:JRB PACKING CO LTD
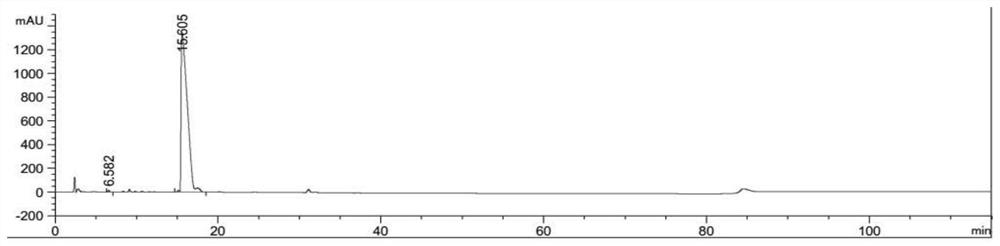
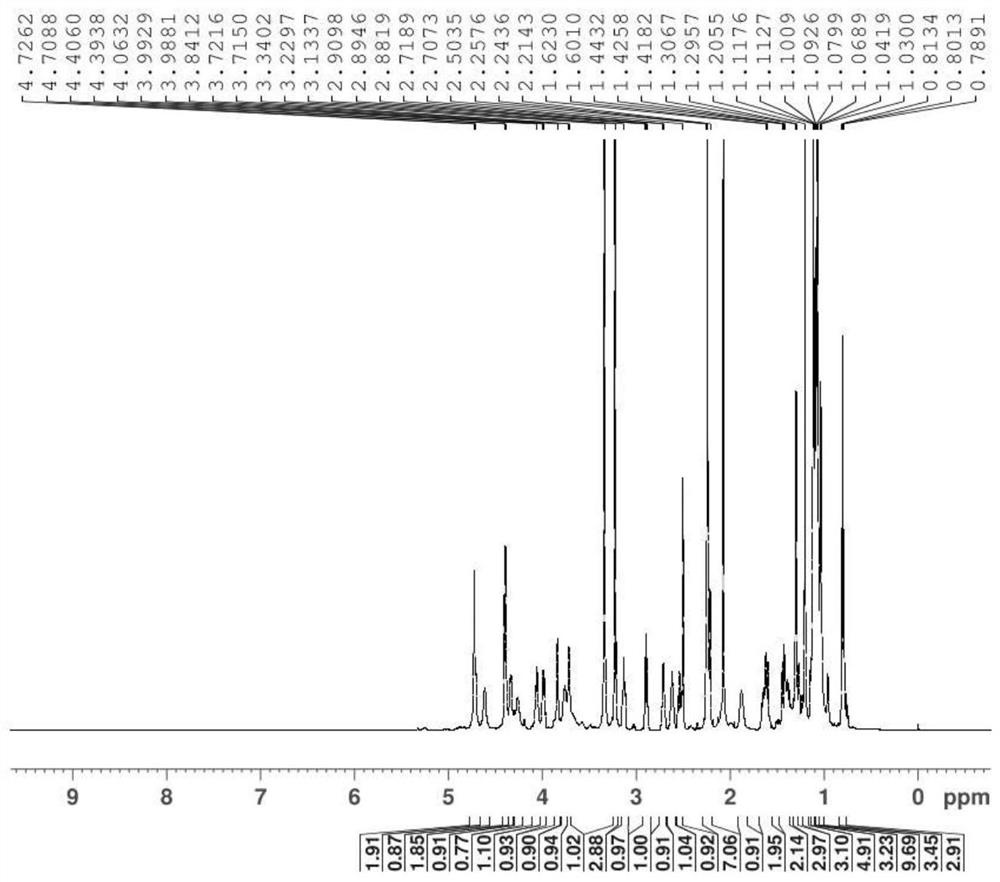
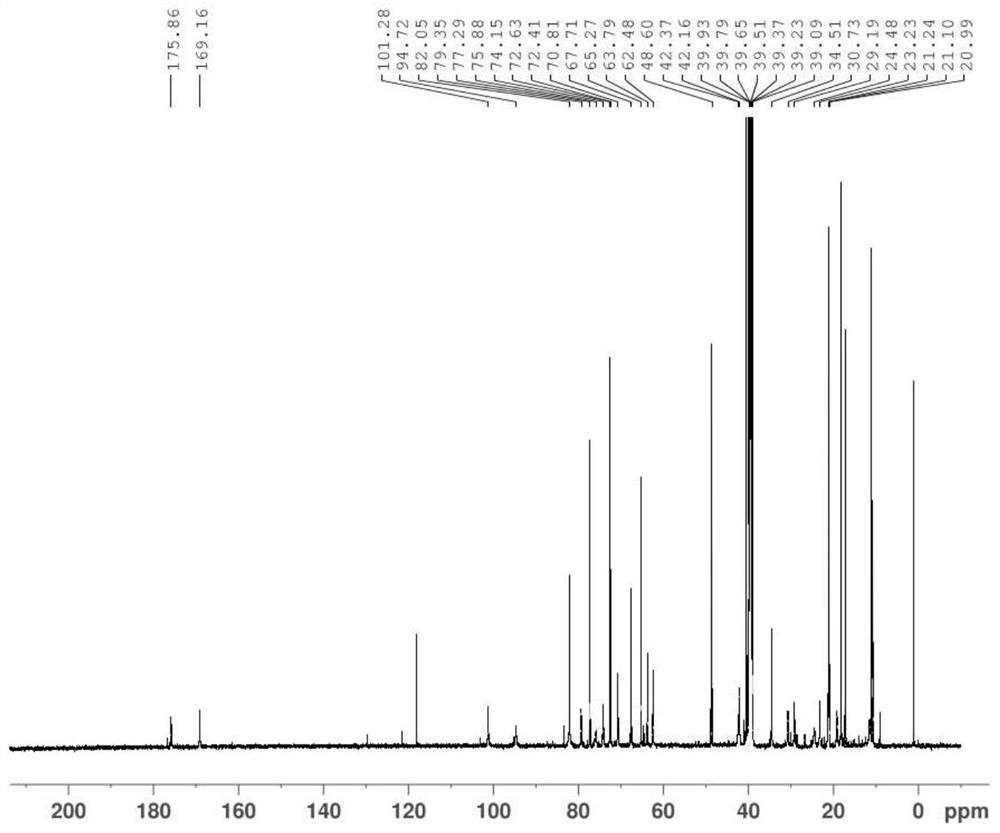
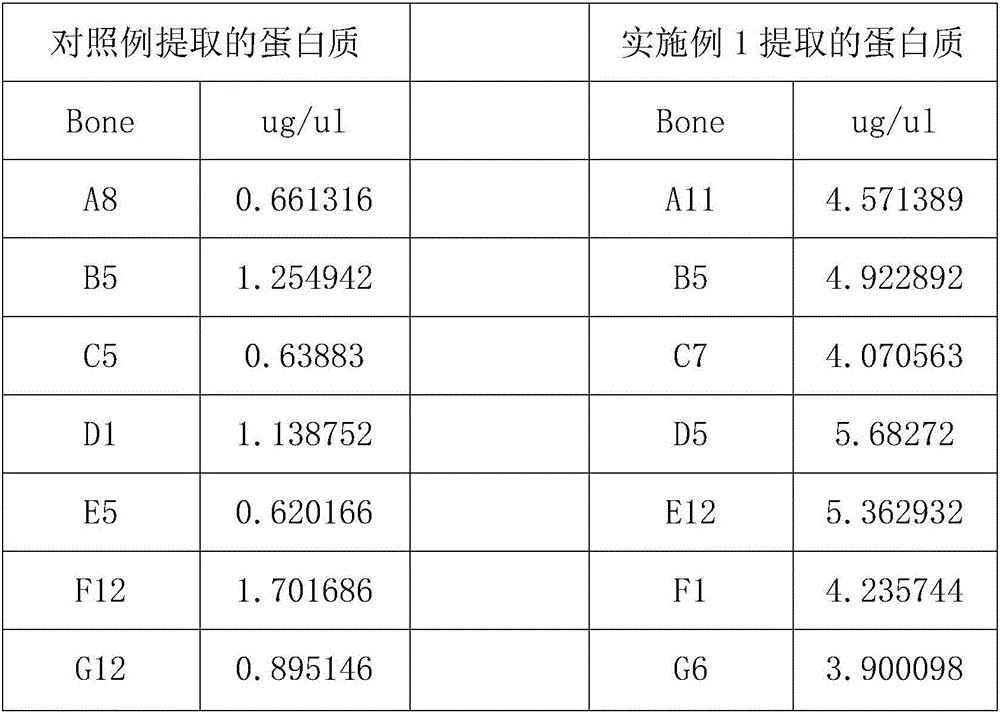
Economic and practical method for extracting and separating bone tissue protein
InactiveCN106749604AIncrease concentrationGuaranteed purityPeptide preparation methodsAnimals/human peptidesBiologyPre cooling
The invention discloses an economic and practical method for extracting and separating bone tissue protein and belongs to the technical field of molecular biology. The method concretely comprises the following steps: placing a weighted centrifugal tube onto ice and pre-cooling; taking a phosphatase inhibitor, a protease inhibitor and phenylmethylsulfonyl fluoride, adding the three into a splitting buffer solution, and then placing the mixture onto the ice and uniformly stirring; weighting 0.3-0.5g of femoral shaft; putting the femoral shaft into a grinding kettle filled with liquid nitrogen and grinding the femoral shaft into powder; transferring the femoral shaft to a spare centrifugal tube and weighting, thereby obtaining the weight of the femoral shaft; multiplying the mass by 1 / 3, and then adding the lysate into the centrifugal tube in concentration of 0.2-0.4g / mL; shaking the mixed solution under a low temperature and then standing; placing the turbid liquid under the conditions of 4 DEG C and 3000-5000rpm and centrifuging; and absorbing the supernate to a new centrifugal tube and then split-charging and storing. According to the invention, the extraction and separation process is simple and the separated protein purity is high, so that the extraction and separation process is suitable for popularization and application in the field of biology.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Moisture and heat resistant high-strength silicone structural adhesive for photovoltaic modules
ActiveCN107880797BExcellent heat and humidity resistanceHumidity has little effectNon-macromolecular adhesive additivesOrganic non-macromolecular adhesivePolymer sciencePtru catalyst
Owner:ZHEJIANG FORST NEW MATERIAL RES INST CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com