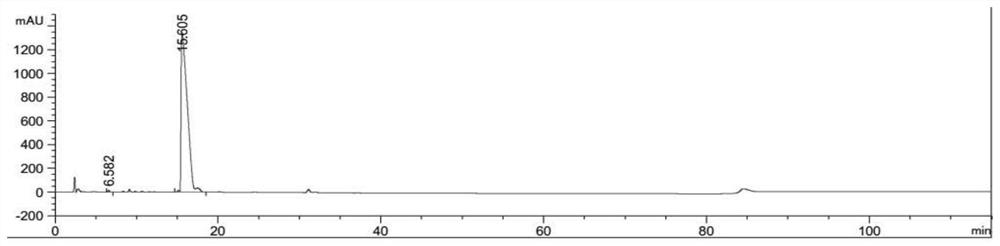
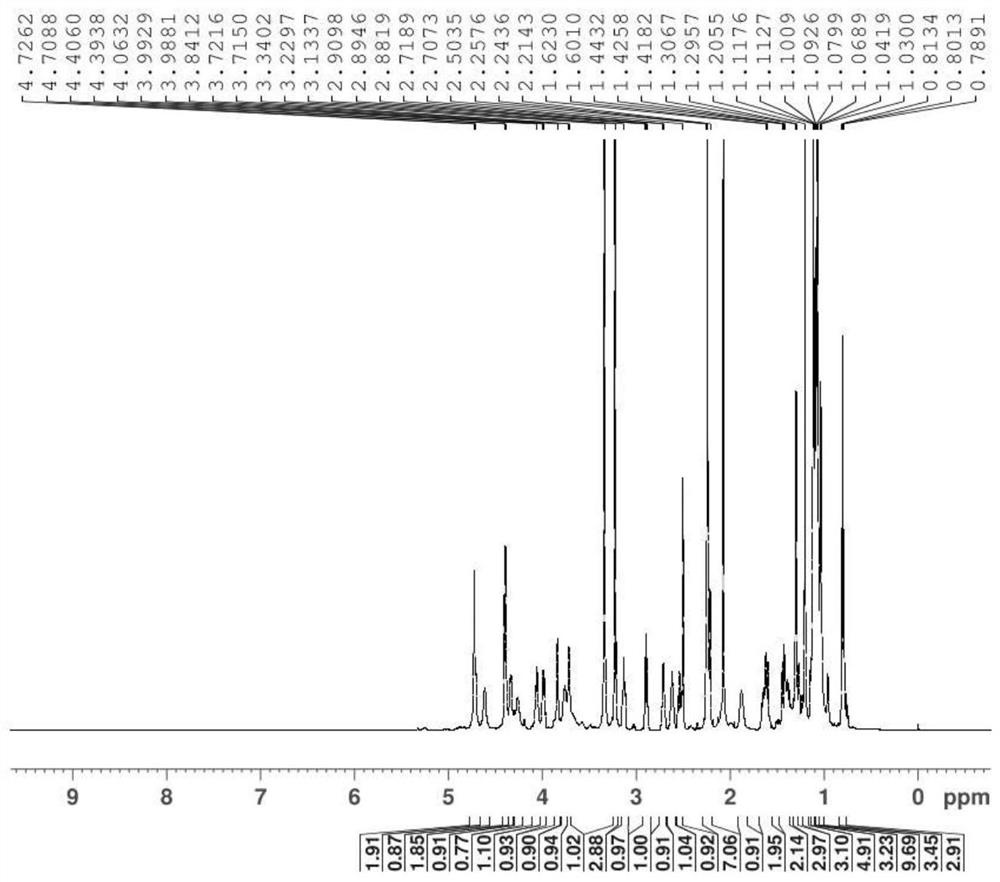
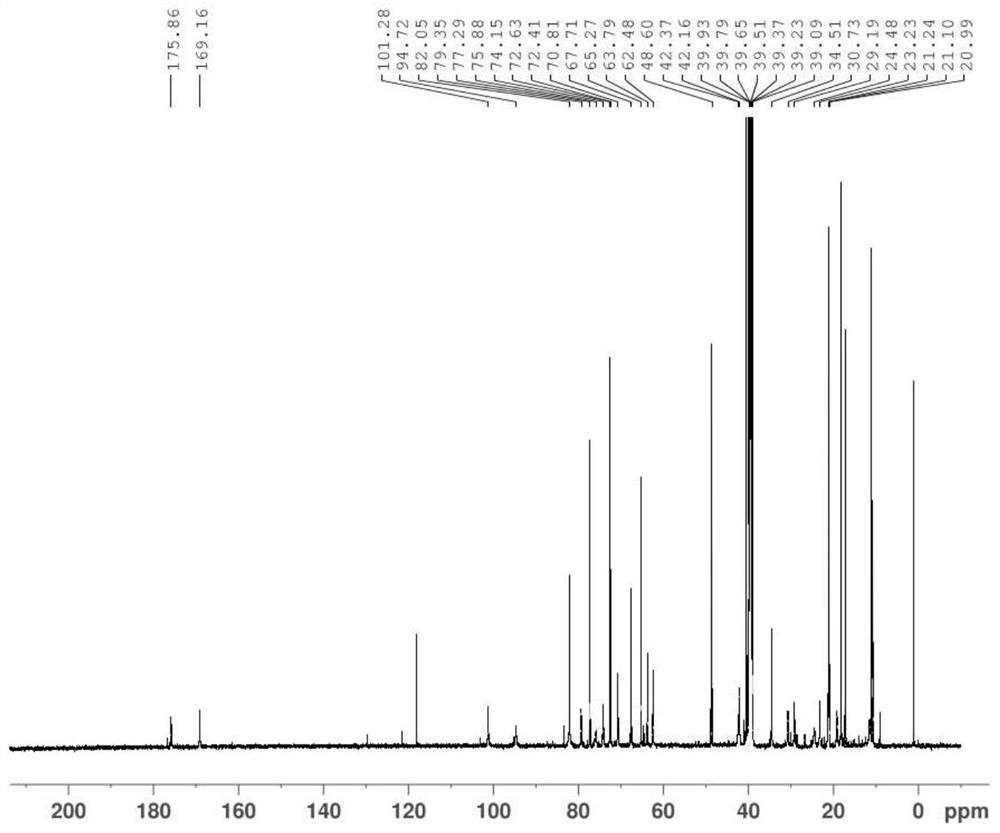
A kind of synthetic method of azithromycin rearrangement impurity r
A technology of azithromycin and a synthesis method is applied in the field of chemistry to achieve the effects of high refining yield, high solubility of raw materials and improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 (1R, 2R, 3R, 6R, 7S, 8S, 9R, 10R, 12R, 15R)-9[[3,4,6-trideoxy-3-(dimethylamino)-β-D-wood -hexapyranosyl]oxy]-3-ethyl-2,10-dihydroxy-7-[(2,6-dideoxy-3-C-methyl-3-O-methyl-a-L-nucleo -hexapyranosyl)oxy]-2,6,8,10,12,15-hexamethyl-4,16-dioxo-14-azabicyclo[11.2.1]hexadecan-13-ene Synthesis of -5-one
[0032] (3R,4R,5S,6R,9R,10S,11S,12R,13R,15R,Z)-12-[[3,4,6-trideoxy-3-(dimethylamino)-β-D- Wood-hexyranosyl]oxy]-6-ethyl-4,5-dihydroxy-10-[(2,6-dideoxy-3-C-methyl-3-O-methyl-a-L- Nucleo-hexapyranosyl)oxy]-3,5,9,11,13,15-hexamethyl-7,16-dioxo-2-azabicyclo[11.2.1]hexadecane-1- Add 20 g of en-8-one (compound II, 26 mmol), 100 ml of dichloromethane into a three-necked flask, control the temperature at 40 ° C, add 4 g of 5% hydrochloric acid, stir for 5 h, add 20 ml of water to wash, separate layers, and take organic The layers were distilled under reduced pressure to obtain 18 g of white solid, with a yield of 90%.
[0033] (1R,2R,3R,6R,7S,8S,9R,10R,12R,15R)-9[[3,4,6-t...
Embodiment 2
[0035] Example 2 (1R, 2R, 3R, 6R, 7S, 8S, 9R, 10R, 12R, 15R)-9[[3,4,6-trideoxy-3-(dimethylamino)-β-D-wood -hexapyranosyl]oxy]-3-ethyl-2,10-dihydroxy-7-[(2,6-dideoxy-3-C-methyl-3-O-methyl-a-L-nucleo -hexapyranosyl)oxy]-2,6,8,10,12,15-hexamethyl-4,16-dioxo-14-azabicyclo[11.2.1]hexadecan-13-ene Synthesis of -5-one
[0036] (3R,4R,5S,6R,9R,10S,11S,12R,13R,15R,Z)-12-[[3,4,6-trideoxy-3-(dimethylamino)-β-D- Wood-hexyranosyl]oxy]-6-ethyl-4,5-dihydroxy-10-[(2,6-dideoxy-3-C-methyl-3-O-methyl-a-L- Nucleo-hexapyranosyl)oxy]-3,5,9,11,13,15-hexamethyl-7,16-dioxo-2-azabicyclo[11.2.1]hexadecane-1- Add 20 g of en-8-one (compound II, 26 mmol), 50 ml of dichloromethane into a three-necked flask, control the temperature at 40 ° C, add 4 g of 5% hydrochloric acid, stir for 5 h, add 20 ml of water to wash, separate layers, and take organic The layers were distilled under reduced pressure to obtain 14 g of white solid, with a yield of 70%.
[0037] (1R,2R,3R,6R,7S,8S,9R,10R,12R,15R)-9[[3,4,6-tr...
Embodiment 3
[0039]Example 3 (1R, 2R, 3R, 6R, 7S, 8S, 9R, 10R, 12R, 15R)-9[[3,4,6-trideoxy-3-(dimethylamino)-β-D-wood -hexapyranosyl]oxy]-3-ethyl-2,10-dihydroxy-7-[(2,6-dideoxy-3-C-methyl-3-O-methyl-a-L-nucleo -hexapyranosyl)oxy]-2,6,8,10,12,15-hexamethyl-4,16-dioxo-14-azabicyclo[11.2.1]hexadecan-13-ene Synthesis of -5-one
[0040] (3R,4R,5S,6R,9R,10S,11S,12R,13R,15R,Z)-12-[[3,4,6-trideoxy-3-(dimethylamino)-β-D- Wood-hexyranosyl]oxy]-6-ethyl-4,5-dihydroxy-10-[(2,6-dideoxy-3-C-methyl-3-O-methyl-a-L- Nucleo-hexapyranosyl)oxy]-3,5,9,11,13,15-hexamethyl-7,16-dioxo-2-azabicyclo[11.2.1]hexadecane-1- Add 20 g of en-8-one (compound II, 26 mmol), 100 ml of dichloromethane into a three-necked flask, control the temperature at 55 ° C, add 4 g of 5% hydrochloric acid, stir for 5 h, add 20 ml of water to wash, separate layers, and take organic The layers were distilled under reduced pressure to obtain 14 g of white solid, with a yield of 70%.
[0041] (1R,2R,3R,6R,7S,8S,9R,10R,12R,15R)-9[[3,4,6-tr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com