Method for directly preparing 2,5-dihydroxymethyl tetrahydrofuran from fructose
A technology of dimethylol tetrahydrofuran and fructose, applied in the direction of organic chemistry, etc., to achieve the effect of inhibiting direct hydrogenation reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
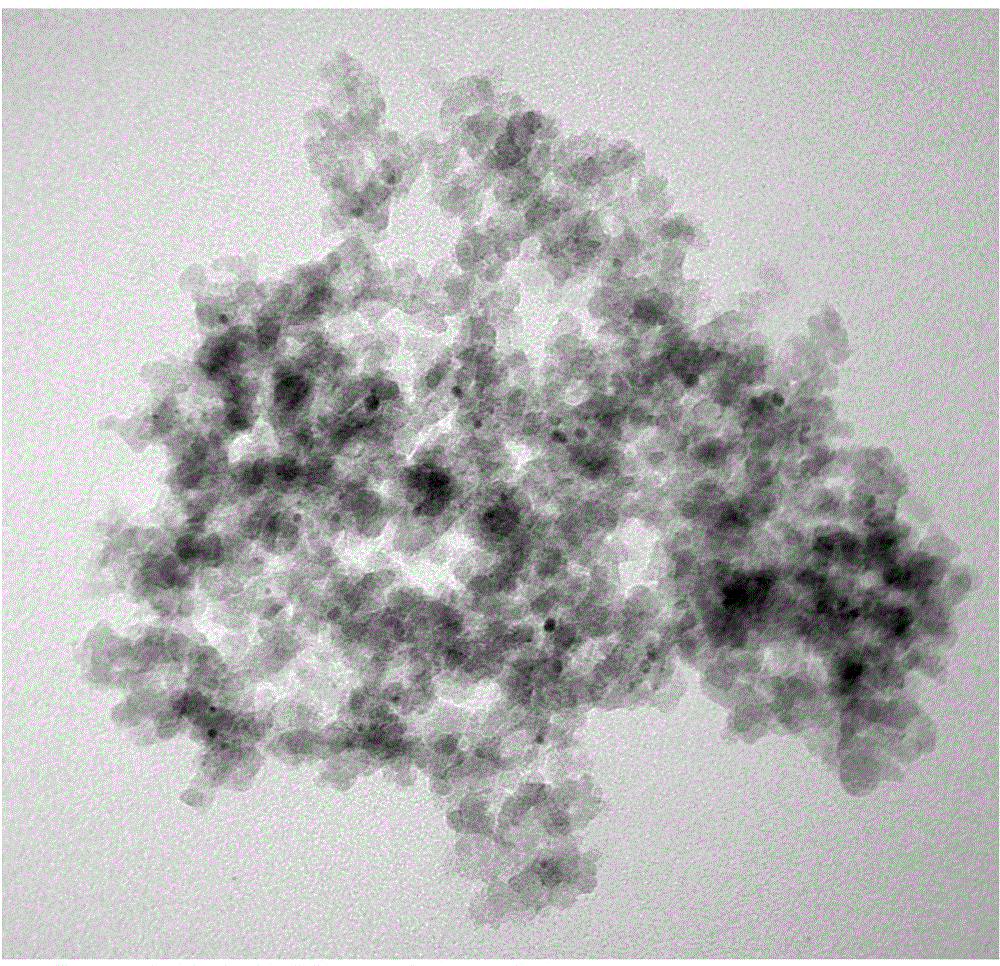
[0016] Ru / SiO 2 -TM catalyst preparation: weigh 4.95g RuCl 3 ·3H 2 O and add 2.70g of water and stir to dissolve. Add 10g of active silicon oxide, stir well to form a paste, let stand for 12h, dry at 120°C, then reduce with hydrogen at 400°C for 2h, 1%O 2 / N 2 Medium passivation 6h. Catalyst transmission electron microscope photo see figure 1 . Put 2 g of the above-mentioned passivated catalyst into a 100 mL beaker, add 40 mL of toluene, 10 mL of trimethylchlorosilane, and 10 mL of pyridine, and reflux for 24 h under nitrogen protection. After washing with ethanol, vacuum-dry.
[0017] The catalytic reaction was carried out in a 50ml stainless steel reactor. 30mg Ru / SiO 2 -TM, 40mg Amberlyst-15, 3mL fructose aqueous solution (2mmol), 6mL cyclohexane were added to a stainless steel autoclave with Teflon lining. After closing the reactor, replace the gas in the reactor with hydrogen four times, control the temperature to 130°C with a temperature controller, fill in hydr...
Embodiment 2-6
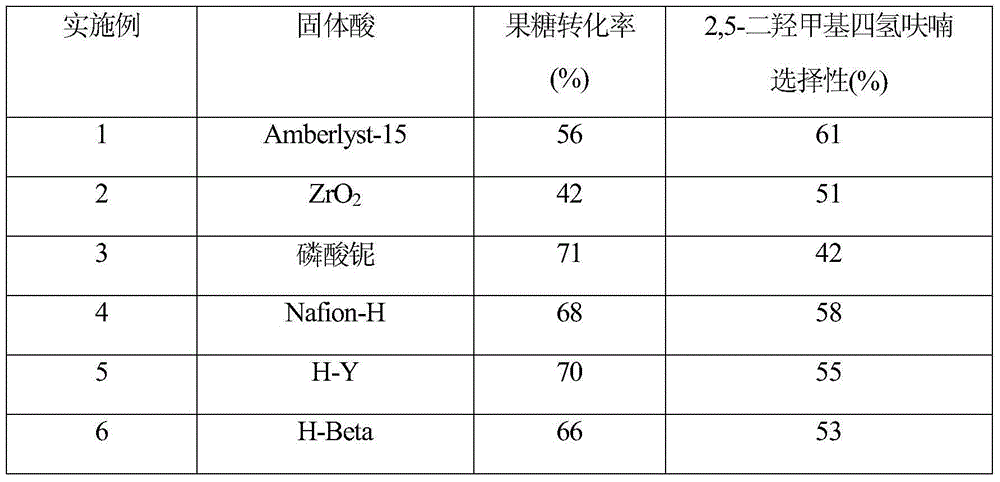
[0019] Effects of different solid acids on the direct preparation of 2,5-dimethyloltetrahydrofuran from fructose. The effects of different types of solid acids on the conversion rate of fructose and the selectivity of 2,5-dimethyloltetrahydrofuran were investigated. ZrO 2 , niobium phosphate, Nafion-H, H-Y molecular sieve and H-Beta molecular sieve replace Amberlyst-15 in embodiment 1 as solid acid catalyst, and others are all the same as embodiment 1. The results are shown in table 1.
[0020] The impact of different solid acids in table 1 on the reaction result
[0021]
[0022] Reaction conditions: 3mL fructose aqueous solution (2mmol), 6mL cyclohexane, Ru / SiO 2 -TM: 30mg, Solid acid: 40mg. Temperature: 130℃, Pressure: 4MPa, Time: 4h.
Embodiment 7-15
[0024] Effect of catalyst components on reaction conversion and selectivity. Catalyst general formula Ru-A-B / SiO 2 -TM, where A and B refer to catalyst promoters. Change the amount of ruthenium trichloride among the embodiment 1 and add respectively different amounts of A, B component nitrates, and others are all the same as embodiment 1. The results are shown in Table 2.
[0025] The impact of table 2 catalyst components on the reaction results
[0026]
[0027] Reaction conditions: 3mL fructose aqueous solution (2mmol), 6mL cyclohexane, Ru-A-B / SiO2 -TM: 30mg, Solid acid: 40mg. Temperature: 130℃, Pressure: 4MPa, Time: 4h. a Refers to the molar ratio of additives to metal ruthenium b Reaction time 12h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com