A kind of azithromycin pharmaceutical composition and preparation method thereof
A technology of azithromycin and a composition, applied in the field of pharmaceutical preparations, can solve problems such as large flavoring agents, and achieve the effects of good taste, good compliance, and increased taking compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
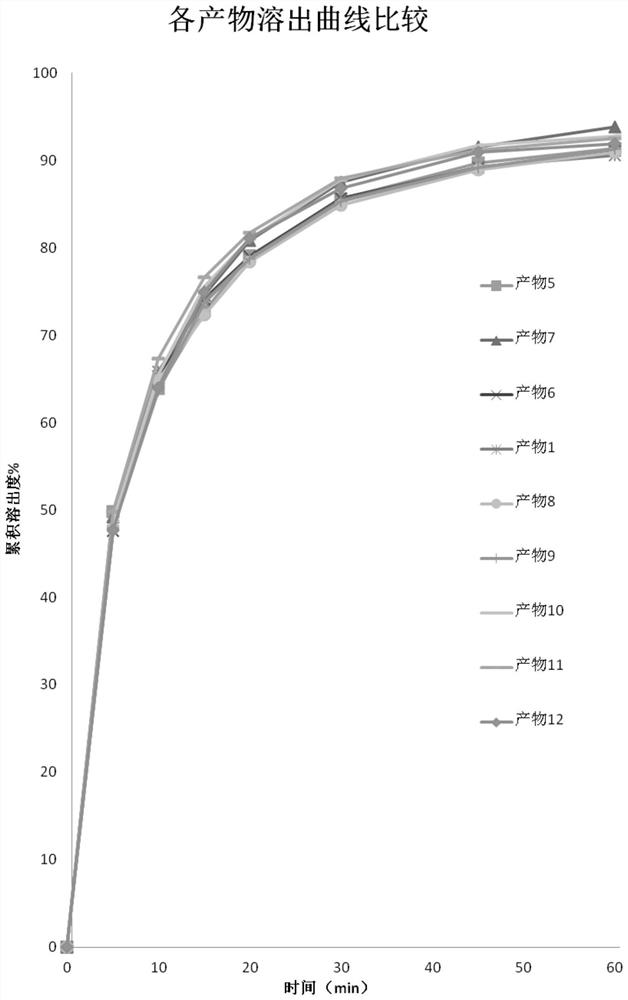
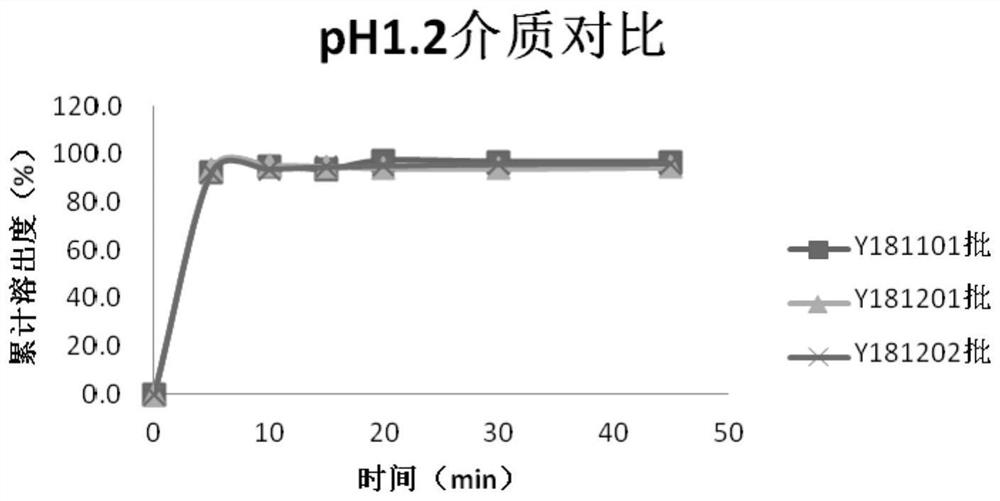
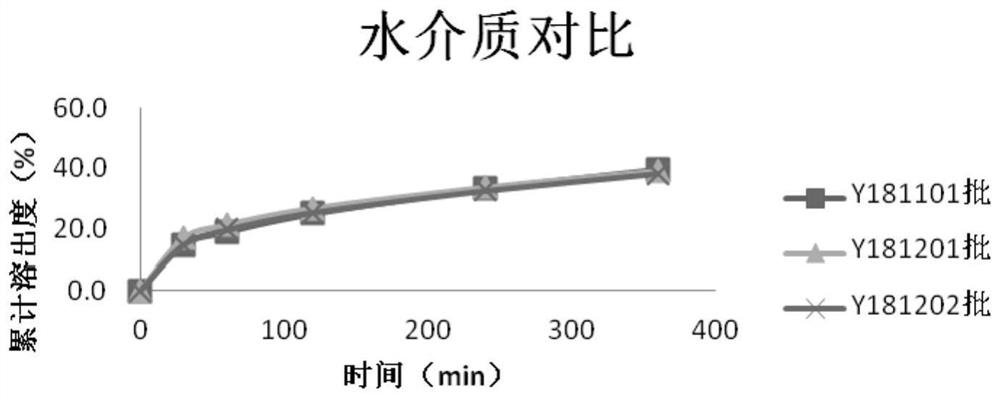
Image
Examples
Embodiment 1
[0073] Example 1 Preparation of the product of azithromycin formulation formulation 1
[0074] Table 1 Recipe 1 Recipe Quantity
[0075]
[0076]
[0077] Preparation Process:
[0078] 1. Pretreatment: sucrose crushed to 120 mesh.
[0079] 2. Mixing: Mix each batch of microcrystalline cellulose, azithromycin, hydroxypropyl cellulose, and sucrose in a high-speed mixing granulator.
[0080] 3. Top spray granulation:
[0081] Add the mixed material into the fluidized bed, set the inlet air temperature to 65°C and the inlet air volume to 20m 3 / h, atomization pressure 1.2kg / cm 2 , peristaltic pump speed 30r / min.
[0082] When the temperature of the material reaches 40°C, the granulation is started, and when the temperature of the material drops to 28°C, the speed of the peristaltic pump is reduced to 10 r / min. The material temperature was maintained at 30-32°C for granulation. When the amount of purified water reaches 450ml, the speed of the peristaltic pump is redu...
Embodiment 2
[0102] Example 2 Preparation of the product of Azithromycin Formulation Formulation 2
[0103] Table 2 prescription 2 formula amount
[0104]
[0105]
[0106] Preparation Process:
[0107] 1. Pretreatment: sucrose crushed to 120 mesh.
[0108] 2. Mixing: Mix each batch of microcrystalline cellulose, azithromycin, hydroxypropyl cellulose, and sucrose in a high-speed mixing granulator.
[0109] 3. Top spray granulation:
[0110] Add the mixed material into the fluidized bed, set the inlet air temperature to 65°C and the inlet air volume to 20m 3 / h, atomization pressure 1.2kg / cm 2 , peristaltic pump speed 30r / min.
[0111] When the temperature of the material reaches 40°C, the granulation is started, and when the temperature of the material drops to 28°C, the speed of the peristaltic pump is reduced to 10 r / min. The material temperature was maintained at 30-32°C for granulation. When the amount of purified water reaches 450ml, the speed of the peristaltic pump is...
Embodiment 3
[0131] Example 3 Preparation of the product of azithromycin formulation formulation 3
[0132] Table 3 prescription 3 formula amount
[0133]
[0134]
[0135] Preparation Process:
[0136] 1. Pretreatment: sucrose crushed to 120 mesh.
[0137] 2. Mixing: Mix each batch of microcrystalline cellulose, azithromycin, hydroxypropyl cellulose, and sucrose in a high-speed mixing granulator.
[0138] 3. Top spray granulation:
[0139] Add the mixed material into the fluidized bed, set the inlet air temperature to 65°C and the inlet air volume to 20m 3 / h, atomization pressure 1.2kg / cm 2 , peristaltic pump speed 30r / min.
[0140] When the temperature of the material reaches 40°C, the granulation is started, and when the temperature of the material drops to 28°C, the speed of the peristaltic pump is reduced to 10 r / min. The material temperature was maintained at 30-32°C for granulation. When the amount of purified water reaches 450ml, the speed of the peristaltic pump is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com