Application of binaphthol derivatives in aspect of active free radical photopolymerization reaction
A technology of free radicals and derivatives, applied in the field of organic photocatalyst catalyzed controllable living radical polymerization, can solve the problems such as the molecular weight growth does not show a good linear trend, the theoretical molecular weight and the actual molecular weight deviation, and the polymer molecular weight control becomes weak. , to achieve the effect of polydispersity, long shelf life and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Photopolymerization experiment of MMA initiated by 3,3'-para-trifluoromethyl substituted phenyl-1,1'-binaphthol / 2-bromophenyl ethyl acetate
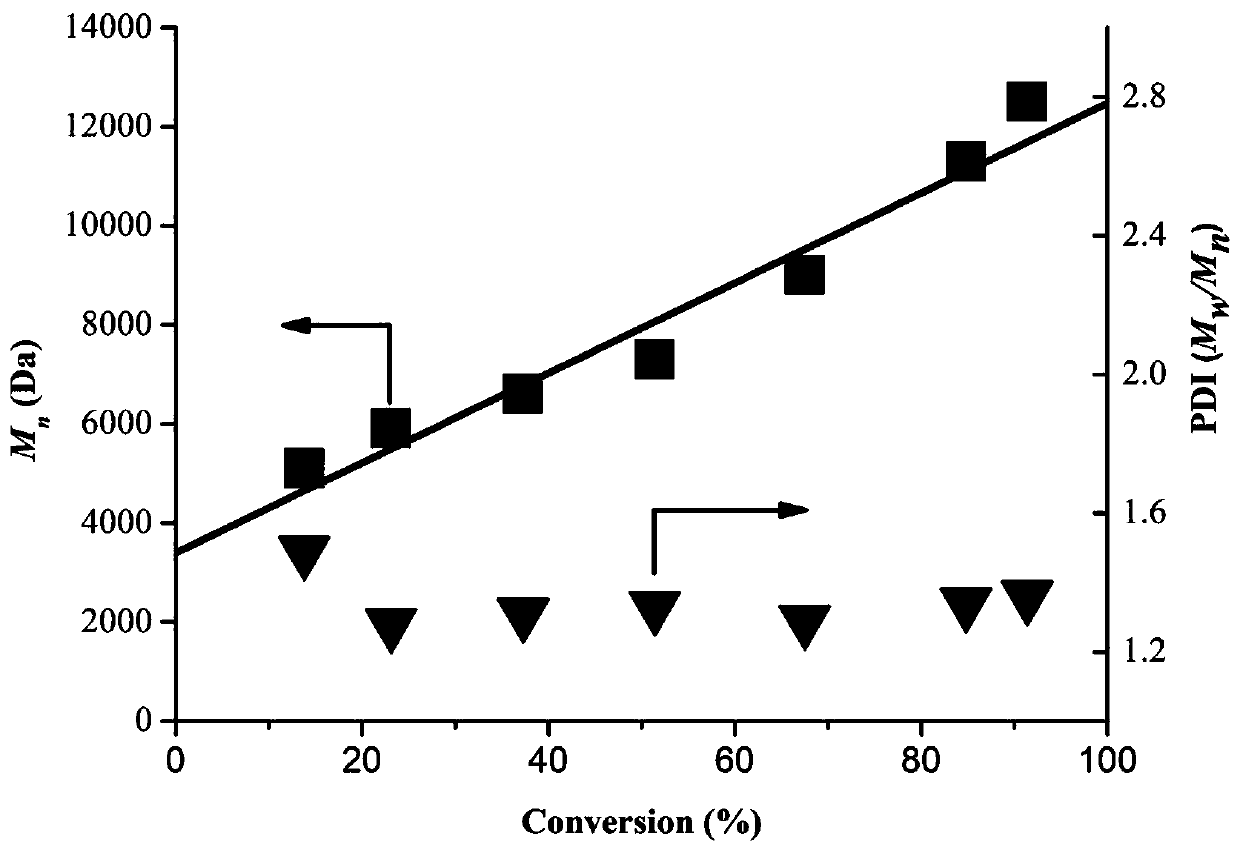
[0034] According to the molar ratio [MMA]:[EBP]:[PC]=100:1:0.1, add the above-mentioned raw materials to a 10 ml Schlenk tube, respectively, the solvent N,N-dimethylacetamide (DMA) and monomer The volume ratio is 1:1. After sealing, the reaction mixture was degassed by freezing and pumping three times, so that the polymerization was carried out in an inert atmosphere. At room temperature, stir the reaction mixture with a magnetic stirrer, and irradiate the mixture with a purple LED (6 W) (control the distance from the center of the reaction tube to the light source to be 2 cm). At a certain time interval, a small amount of the reaction mixture was added to deuterated chloroform containing BHT (250 ppm) to terminate the polymerization, the conversion rate was monitored by nuclear magnetism, the remaining reaction liquid was...
Embodiment 2
[0041] According to the molar ratio [MMA]:[EBP]:[PC]=100:0.5:0.01, 100:0.5:0.02 and 100:2:0.05 do not add the above raw materials to a 10 ml Schlenk tube, solvent N, N-two The volume ratio of methylacetamide (DMA) to monomer is 1:1, sealed, and the reaction mixture is degassed by refrigerating and pumping three times, so that the polymerization proceeds in an inert atmosphere. Refer to Embodiment 1 for other operations.
[0042] Under the condition of [MMA]:[EBP]:[PC]=100:0.5:0.01, the reaction time was 5h, the monomer conversion rate reached 72.2%, the Mn=15900, PDI=1.53 of the polymerized product.
[0043] Under the condition of [MMA]:[EBP]:[PC]=100:0.5:0.02, reacted for 5h, the monomer conversion rate reached 74.3%, the polymerized product had Mn=16200, PDI=1.46.
[0044] Under the condition of [MMA]:[EBP]:[PC]=100:2:0.05, reacted for 5h, the monomer conversion rate reached 80.5%, the polymerized product had Mn=8000, PDI=1.20.
[0045] It shows that as the ratio of monomer and ini...
Embodiment 3
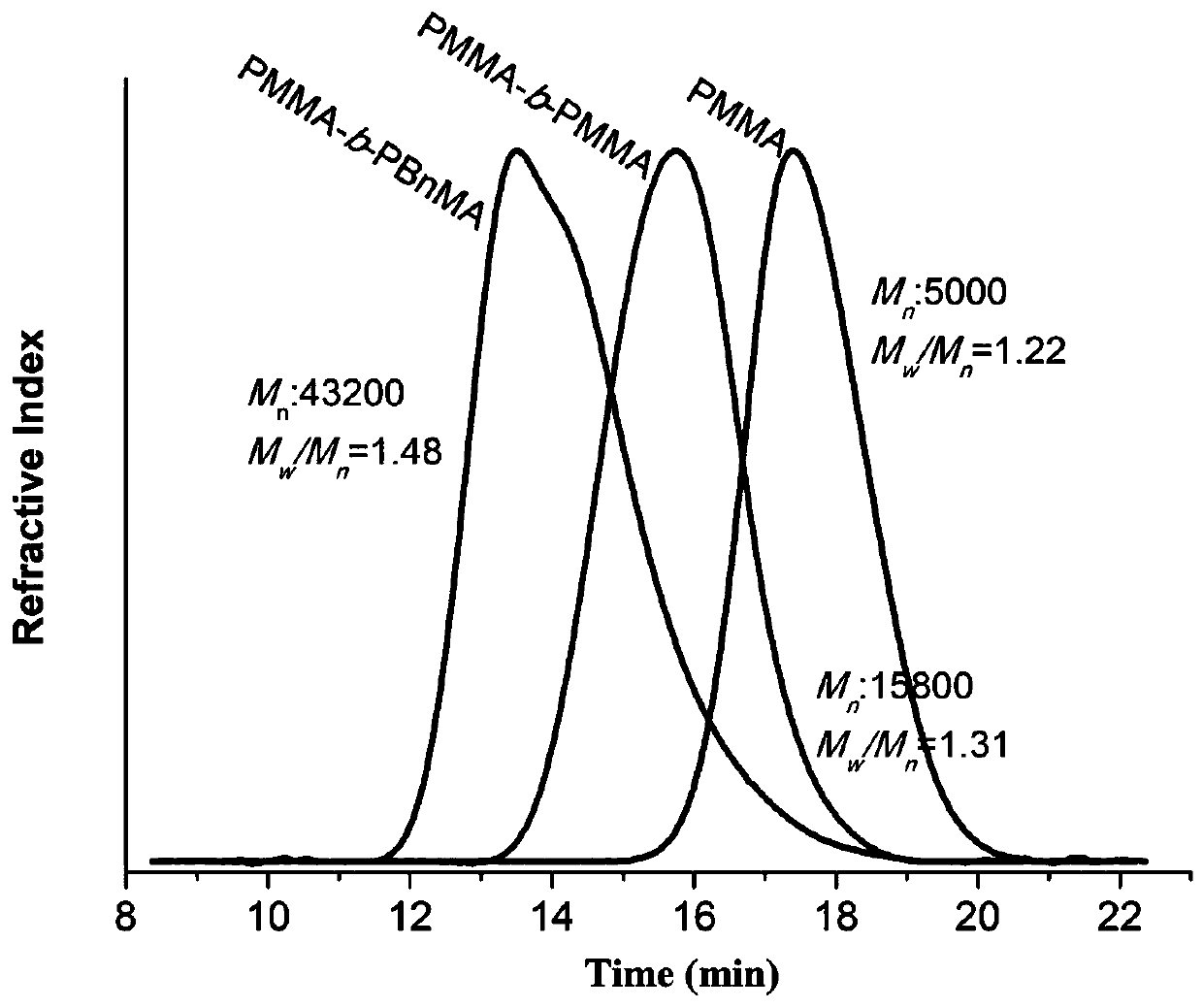
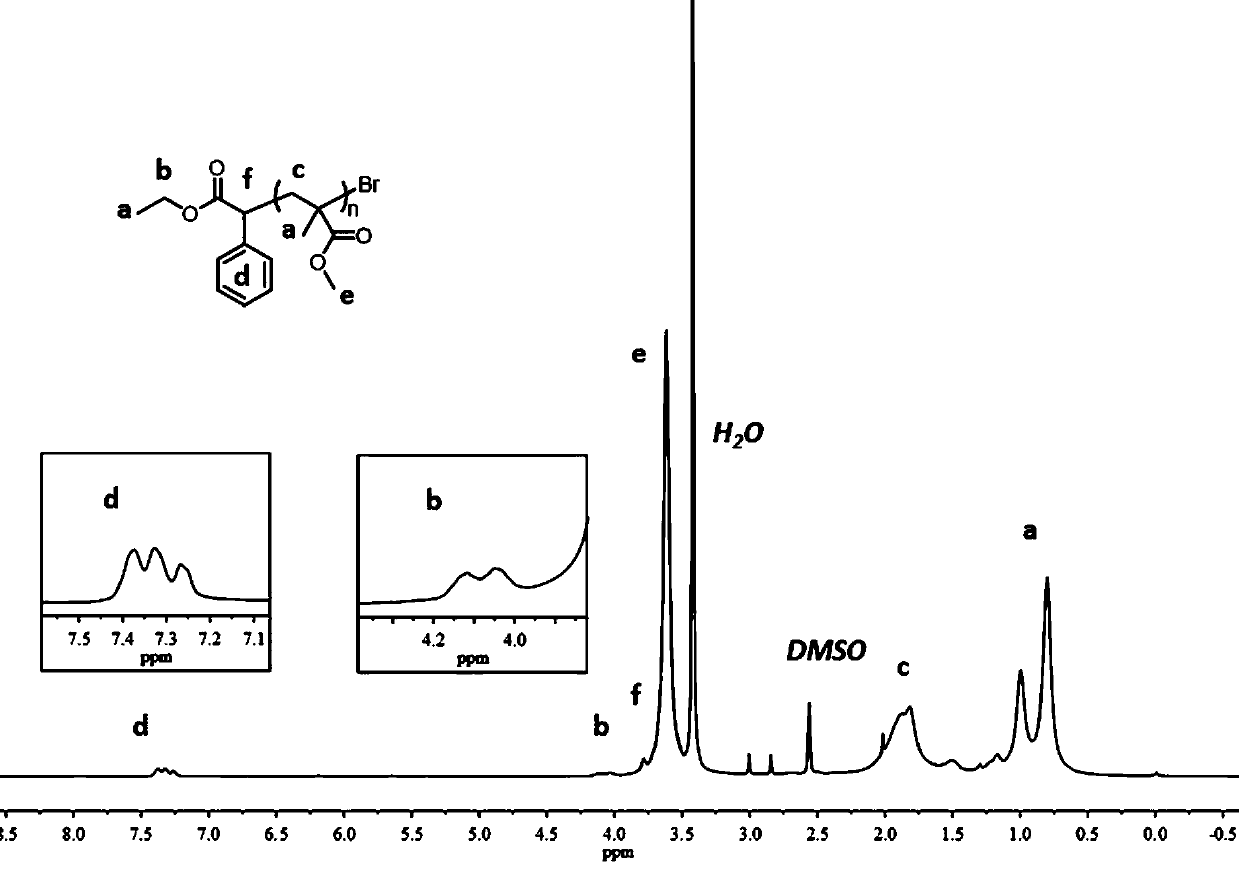
[0046] Example 3 Preparation of PMMA-Br macroinitiator
[0047] Dissolve MMA (2.00 mL, 18.8 mmol, 1000 equivalents), EBP (65.6 μL, 376 μmol, 20 equivalents) and photocatalyst (18.8 μmol, 1 eq.) in 3.00mL DMA, add the above raw materials to a 10 ml Schlenk tube , Sealed, and degassed the reaction mixture by refrigerating and pumping three times to make the polymerization proceed in an inert atmosphere. Refer to Embodiment 1 for other operations. After 4 hours of reaction, the reaction solution was poured into 150 mL of methanol and stirred for 5 hours. The resulting precipitate was then separated by vacuum filtration and washed with an appropriate amount of methanol. Then the polymer was re-dissolved in the minimum amount of DMA, poured into 100 mL methanol again to settle, and stirred for 3 hours. The product was collected again by vacuum filtration, and dried under reduced pressure to obtain a white powder (Mn = 5.00kDa, PDI = 1.22), and the obtained PMMA polymer was dissolve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com