Application of Binaphthol Derivatives in Living Radical Photopolymerization
A technology of derivatives and free radicals, which is applied in the field of organic photocatalysts to catalyze controllable active free radical polymerization, can solve problems such as the molecular weight growth does not show a good linear trend, the deviation between theoretical molecular weight and actual molecular weight, and the weakening of polymer molecular weight control. , to achieve the effects of controllable molecular weight, long modification and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 3,3'-para-trifluoromethyl substituted phenyl-1,1'-binaphthol / ethyl 2-bromophenylacetate initiated photopolymerization experiment of MMA
[0034]According to the molar ratio [MMA]:[EBP]:[PC]=100:1:0.1, add the above raw materials into 10 ml Schlenk tube respectively, the volume of solvent N,N-dimethylacetamide (DMA) and monomer The ratio is 1:1. Sealed and the reaction mixture was degassed by refrigerated pumping three times, allowing the polymerization to proceed under an inert atmosphere. At room temperature, the reaction mixture was well stirred with a magnetic stirrer, and the mixture was irradiated by a purple LED (6 W) (the distance from the center of the reaction tube to the light source was controlled to be 2 cm). At a certain time interval, take a small amount of the reaction mixture and add it to deuterated chloroform containing BHT (250ppm) to terminate the polymerization, monitor the conversion rate by NMR, settle the remaining reaction solution in...
Embodiment 2
[0041] According to the molar ratio [MMA]:[EBP]:[PC]=100:0.5:0.01, 100:0.5:0.02 and 100:2:0.05, add the above raw materials into 10 ml Schlenk tube, solvent N,N-di The volume ratio of methylacetamide (DMA) to monomer was 1:1, sealed, and the reaction mixture was degassed by cryo-pumping three times, so that the polymerization was carried out in an inert atmosphere. Refer to Embodiment 1 for other operations.
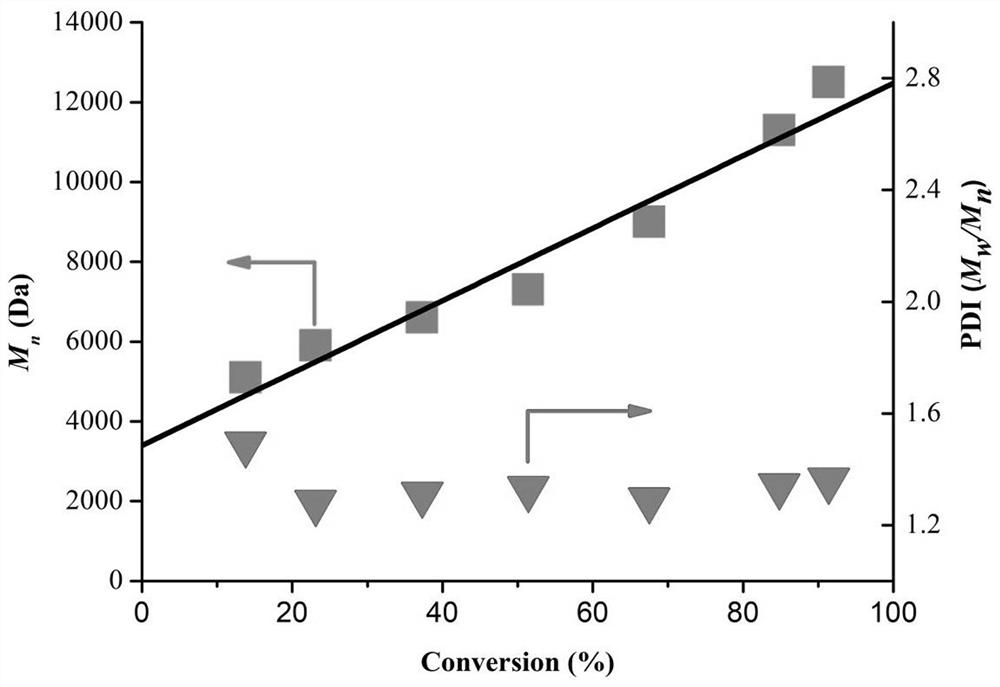
[0042] Under the condition of [MMA]:[EBP]:[PC]= 100:0.5:0.01, after 5 hours of reaction, the monomer conversion rate reached 72.2%, and the Mn=15900 and PDI=1.53 of the polymerization product.
[0043] Under the condition of [MMA]:[EBP]:[PC]= 100:0.5:0.02, after 5 hours of reaction, the monomer conversion rate reached 74.3%, and the Mn=16200 and PDI=1.46 of the polymerization product.
[0044] Under the condition of [MMA]:[EBP]:[PC]= 100:2:0.05, after 5 hours of reaction, the monomer conversion rate reached 80.5%, and the Mn=8000 and PDI=1.20 of the polymerization produ...
Embodiment 3
[0046] The preparation of embodiment three PMMA-Br macroinitiators
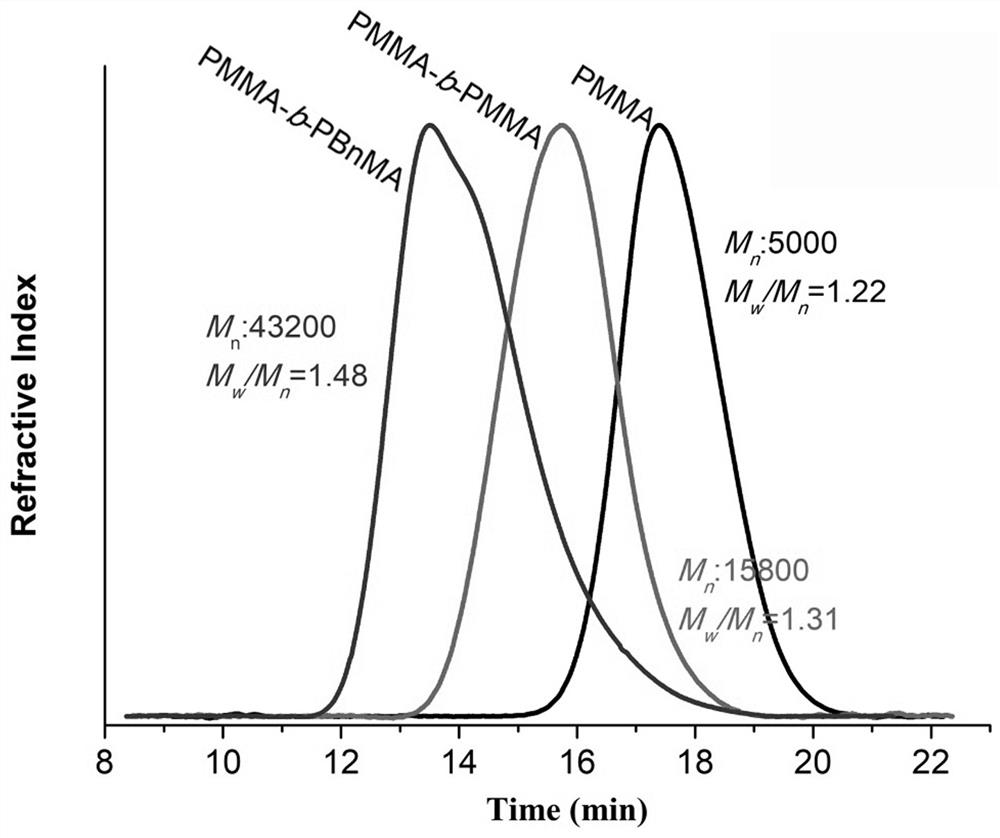
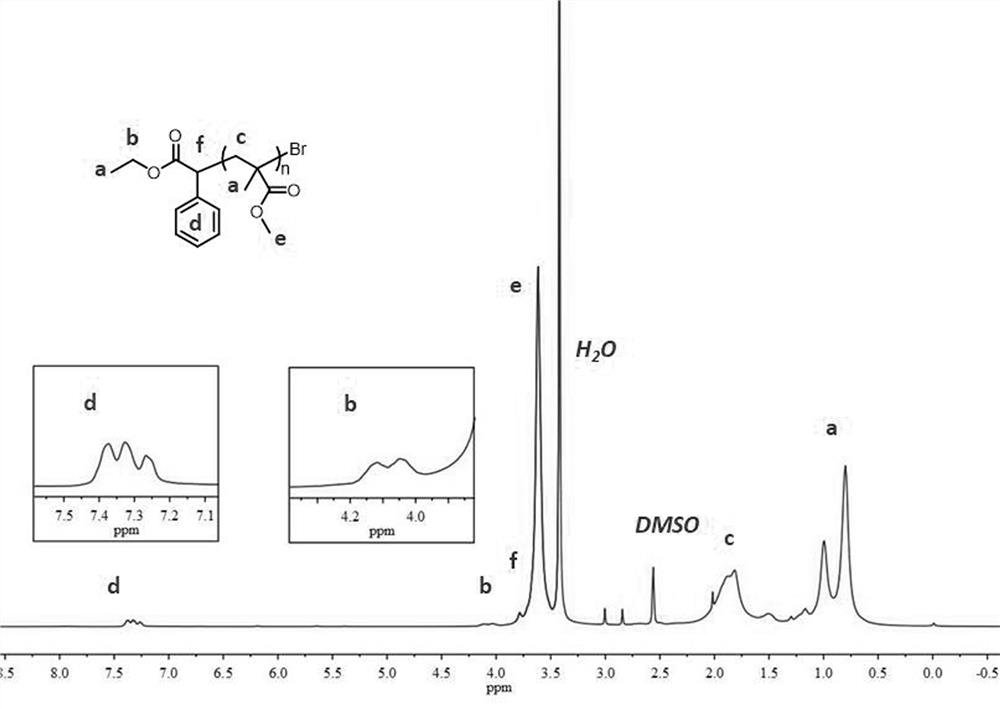
[0047] Dissolve MMA (2.00 mL, 18.8 mmol, 1000 eq.), EBP (65.6 μL, 376 μmol, 20 eq.) and photocatalyst (18.8 μmol, 1 eq.) in 3.00 mL DMA and add the above materials into a 10 ml Schlenk tube , sealed, and the reaction mixture was degassed by cryo-evacuation three times, allowing the polymerization to proceed under an inert atmosphere. Refer to Embodiment 1 for other operations. After reacting for 4 hours, the reaction solution was poured into 150 mL methanol and stirred for 5 hours. The resulting precipitate was then isolated by vacuum filtration and washed with an appropriate amount of methanol. The polymer was then redissolved in a minimal amount of DMA, settled again by pouring into 100 mL of methanol, and stirred for 3 hours. The product was collected again by vacuum filtration and dried under reduced pressure to obtain a white powder (Mn = 5.00 kDa, PDI = 1.22), and the obtained PMMA polymer was dissol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com