A kind of methysergide maleate injection and preparation method thereof
A technology of methysergide maleate and injection, which can be used in medical formulas, medical preparations of non-active ingredients, drug combinations, etc., and can solve problems such as boosting blood pressure and vomiting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
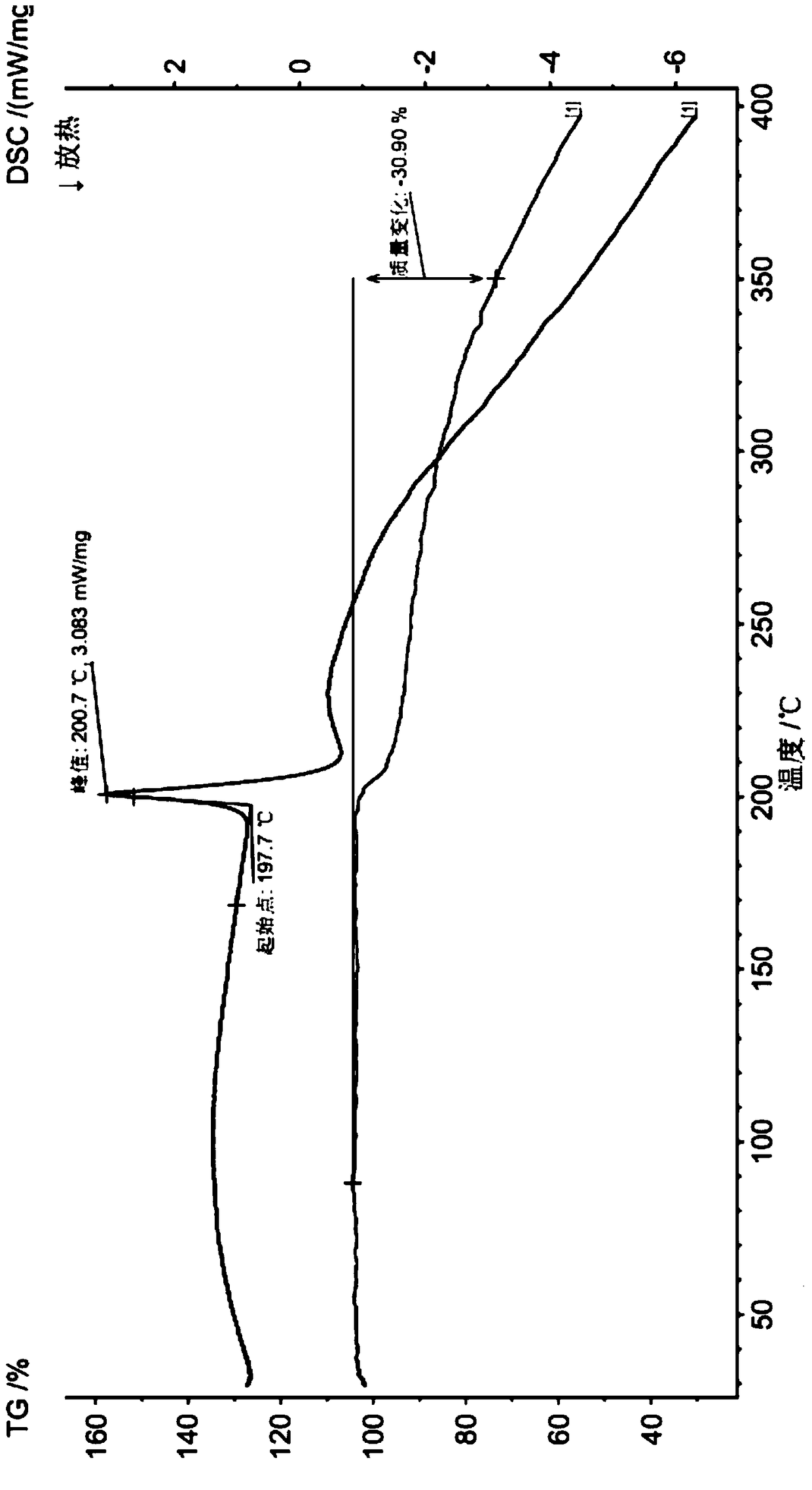
[0034] The preparation of the methysergide maleate used in the injection of the present invention in embodiment 1
[0035]Weigh 10g of crude methylergonovine maleate, add 30g of methanol, and 120g of ethanol into a 250mL single-port reaction flask in turn, heat to 60°C to dissolve; add 1g of activated clay, stir for 30 minutes, filter while it is hot, and cool the filtrate to 0°C with stirring , separated by filtration to obtain a solid, and dried to obtain 8.2 g of methysergide maleate, with a yield of 82%.
[0036] The HPLC peak area normalized method content was 99.88%.
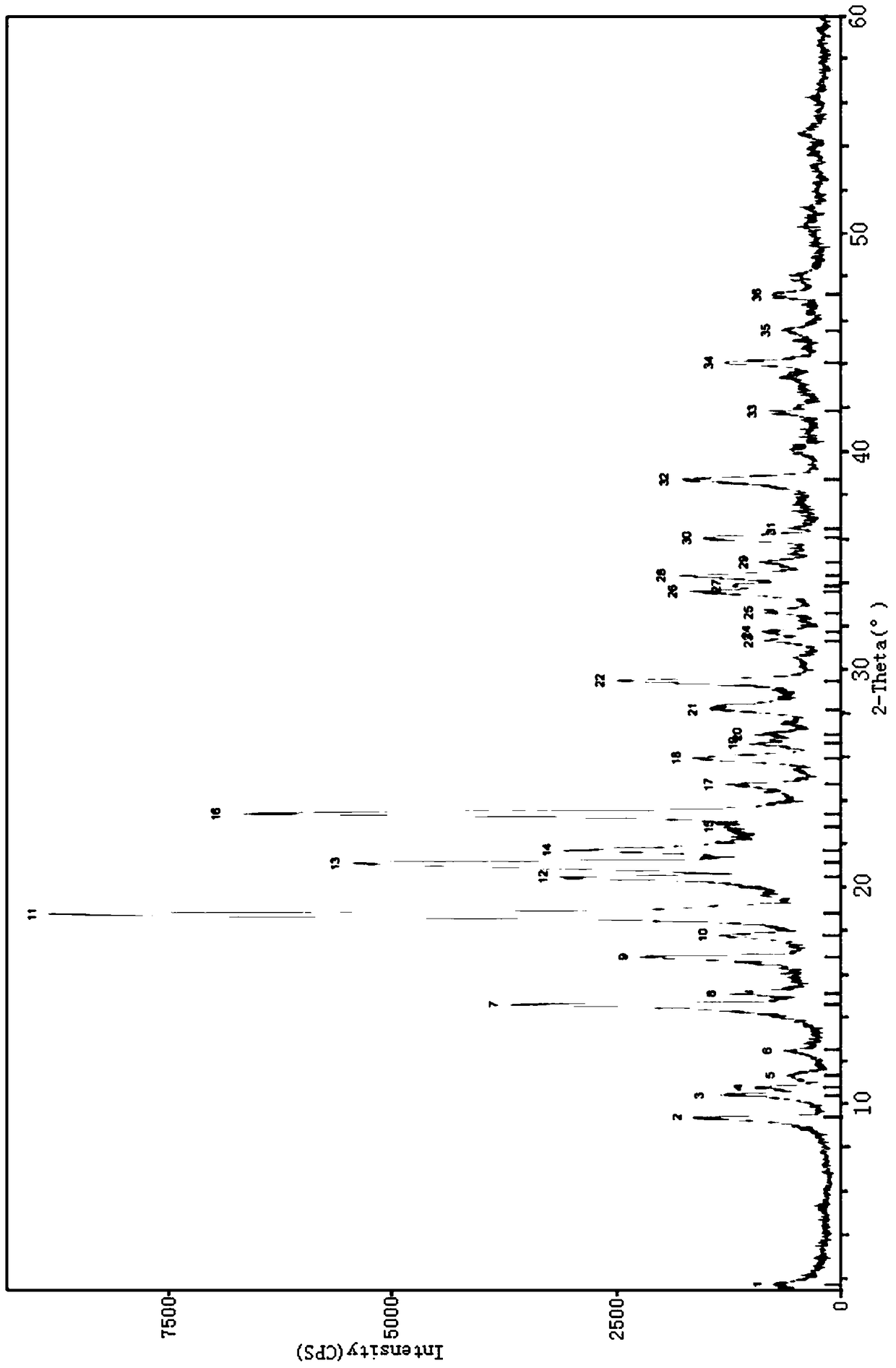
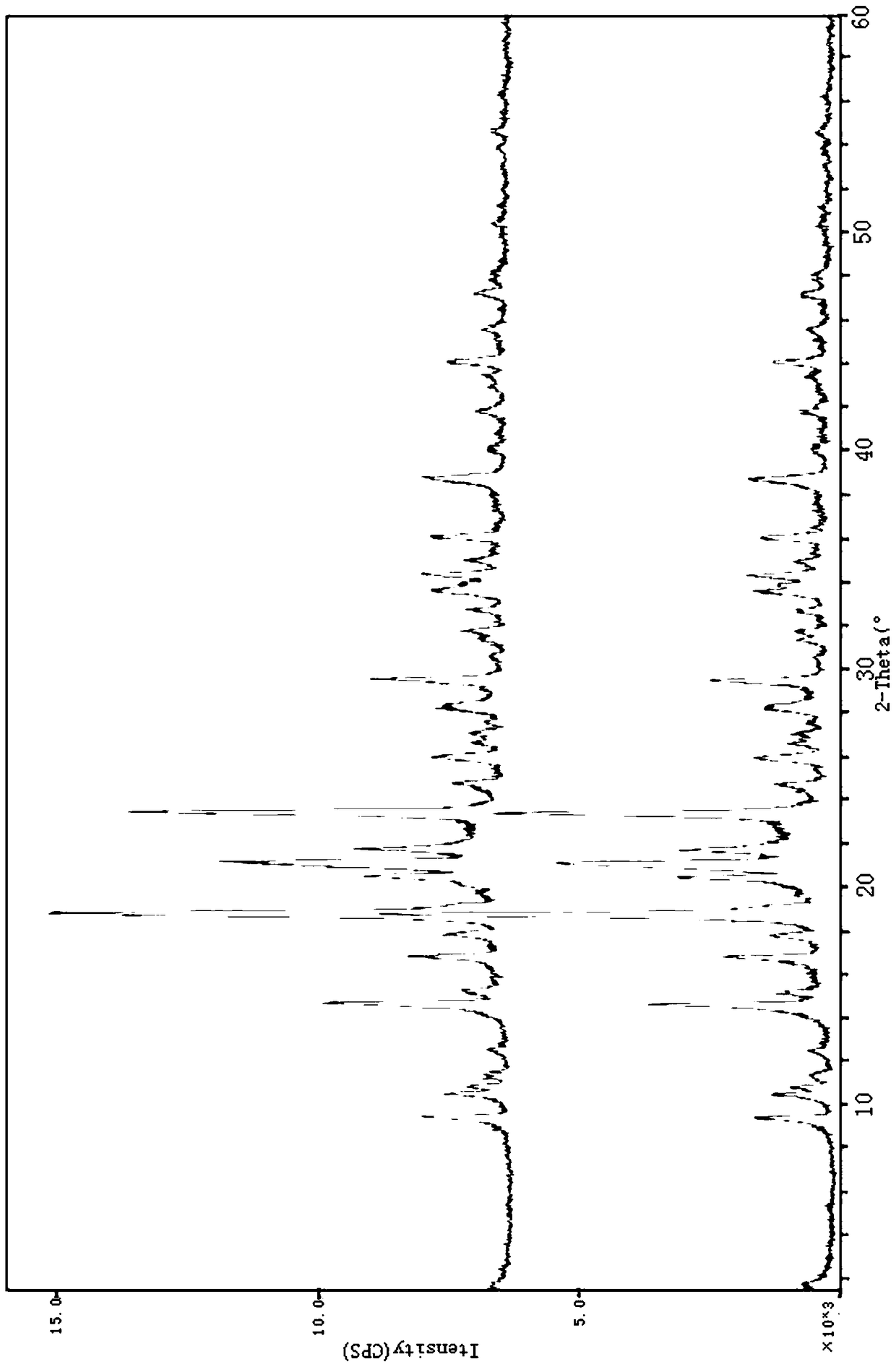
[0037] Carry out X-ray powder diffraction to the methysergide maleate prepared in the present embodiment 1, use Cu-Ka radiation, use the X-ray powder diffraction that 2θ angle represents at 9.459 °, 17.820 °, 18.740 °, 21.159 ° There are characteristic peaks; further, there are characteristic peaks at 10.861°, 15.219°, 20.920°, 21.801°, 23.680°, 27.079°, 28.538° in the X-ray powder diffraction represented...
Embodiment 2
[0040] The preparation of the methysergide maleate used in the injection of the present invention in embodiment 2
[0041] Weigh 10g of methysergide maleate, 25g of methanol, and 25g of ethanol into a 250mL single-port reaction flask in turn, heat to 50°C to dissolve; add 0.5g of activated clay, stir for 30 minutes, filter while hot, and cool the filtrate to 5°C , separated by filtration to obtain a solid, and dried to obtain 7.3 g of methysergide maleate, with a yield of 73%.
[0042] The HPLC peak area normalized method content was 99.87%.
Embodiment 3
[0043] The preparation of the methysergide maleate used in the injection of the present invention in embodiment 3
[0044] Weigh 10g of methysergide maleate, 45g of methanol, and 450g of ethanol into a 1000mL single-port reaction flask in turn, heat to 70°C to dissolve; add 2g of activated clay, stir for 30 minutes, filter while hot, and cool the filtrate to -5°C , separated by filtration to obtain a solid, and dried to obtain 6.9 g of methysergide maleate, with a yield of 69%.
[0045] The HPLC peak area normalized method content was 99.88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com