Pharmaceutical composition for treating steatohepatitis and preparation method of pharmaceutical composition
A steatohepatitis and composition technology, which is applied in the field of pharmaceutical preparations, can solve the problems of elimination of candidate drugs for solubilization requirements, and achieve the effects of avoiding drug degradation reactions, improving dissolution rate, and avoiding dust pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
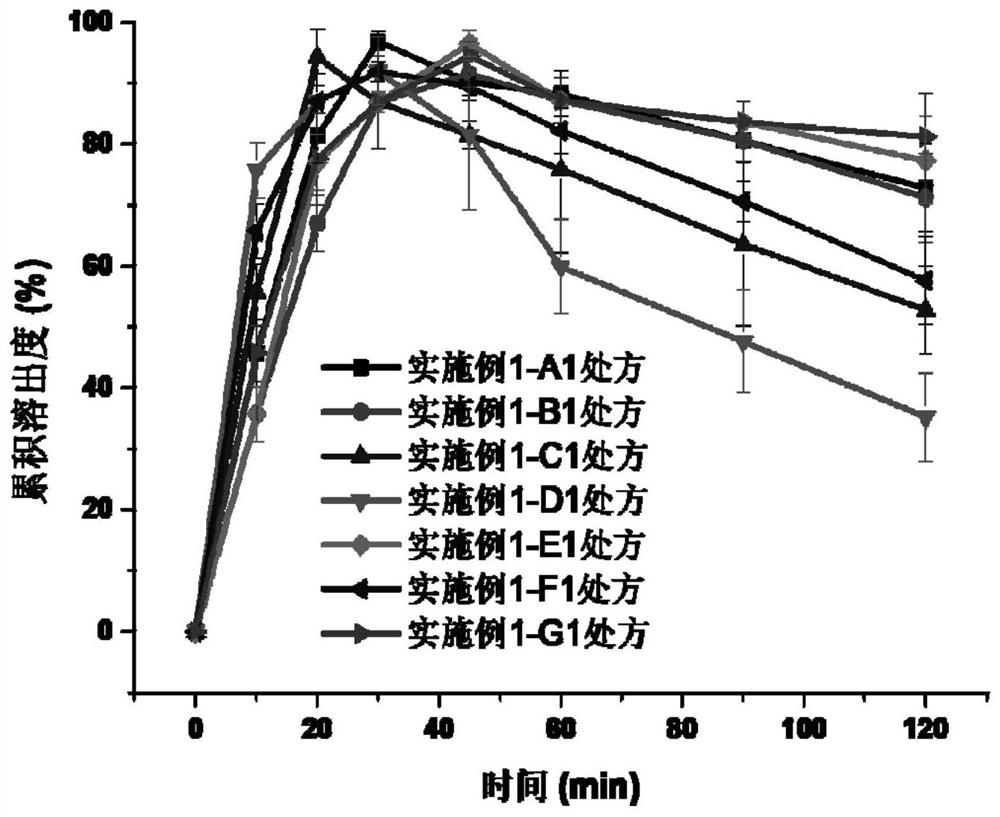
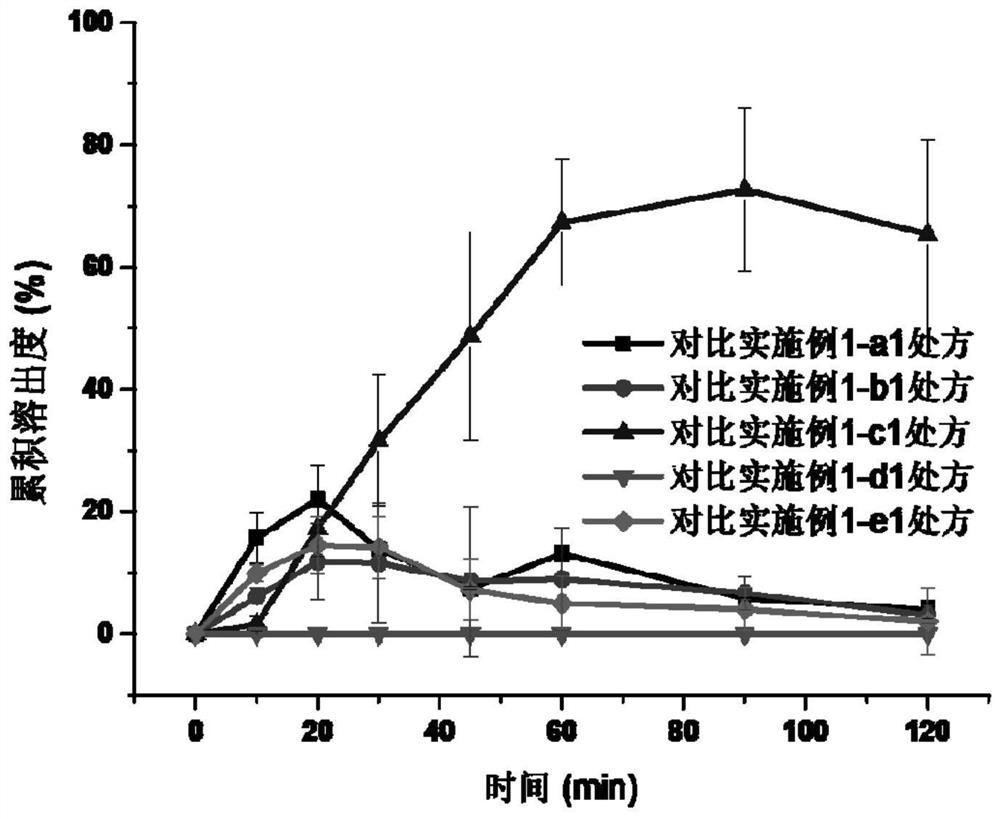
Embodiment 1
[0046] Prescription composition:
[0047] Table 1. The composition of the prescription of embodiment 1
[0048]
[0049]
[0050] Preparation Process:
[0051] 1. Prepare a blank matrix: add polyethylene glycol, poloxamer 188, and anhydrous citric acid in sequence in order of melting point from low to high at a temperature 5-15°C higher than the melting temperature of the carrier;
[0052] 2. Remove air bubbles: stand still, ultrasonic or negative pressure to remove air bubbles;
[0053] 3. Add the compound shown in formula (I): Add the compound drug substance shown in formula (I) under stirring state, continue to stir to make it completely melt in the matrix;
[0054] 4. Capsule filling: Transfer the prepared melted content to the preheated insulation barrel of the capsule filling machine, turn on the stirring function, and pour the melted content into the gelatin hard capsule with the preset filling parameters (control the average The difference in filling capacity ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com