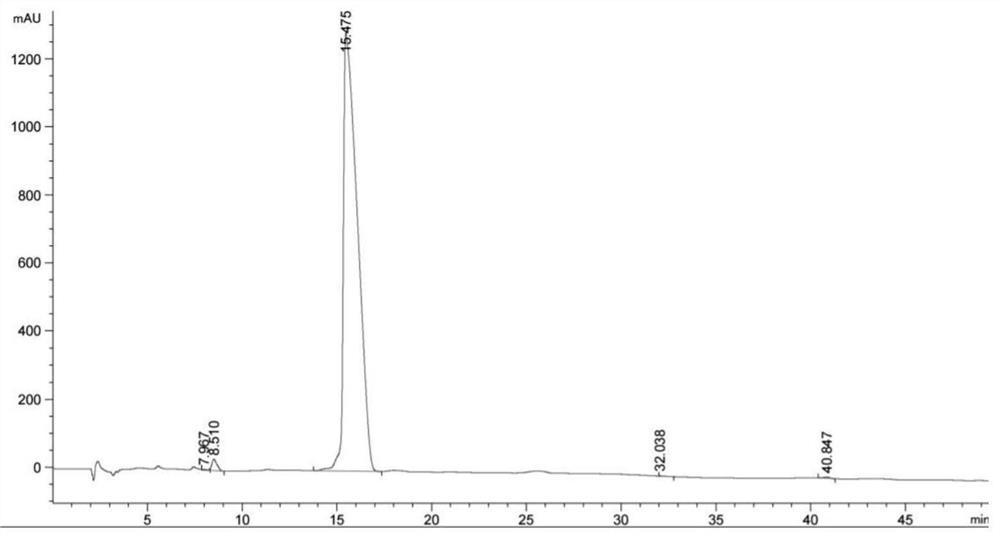
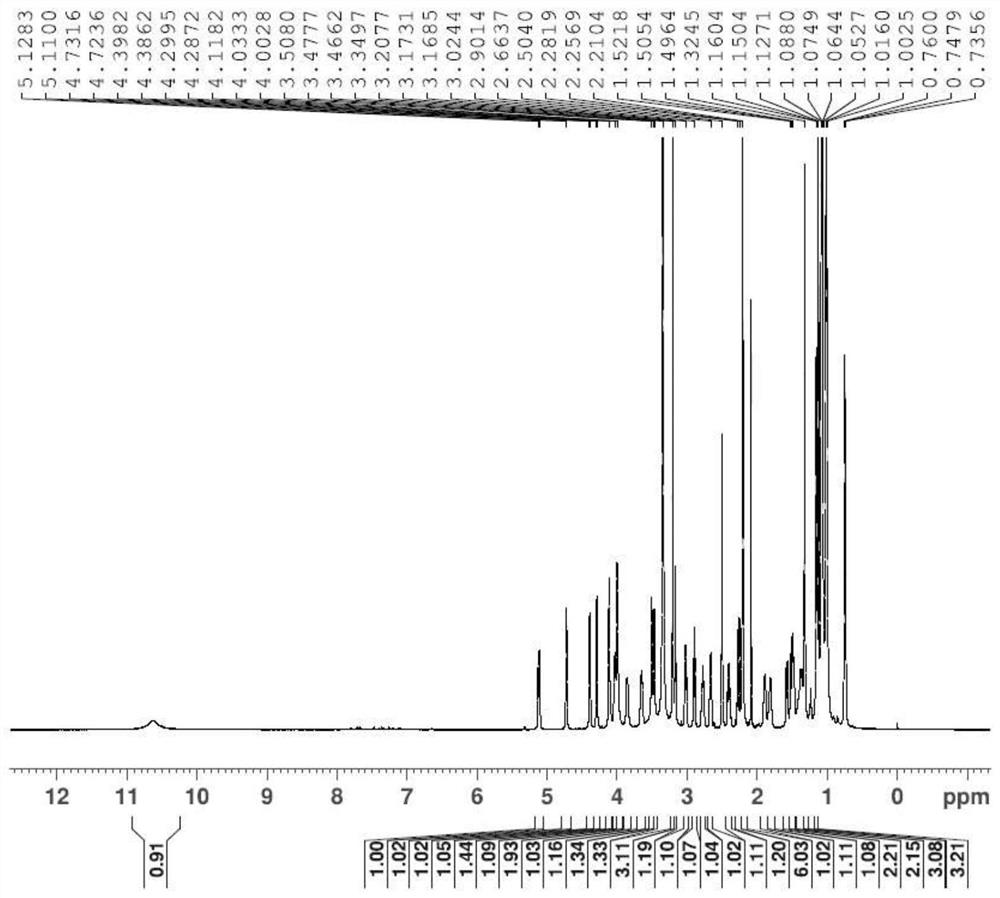
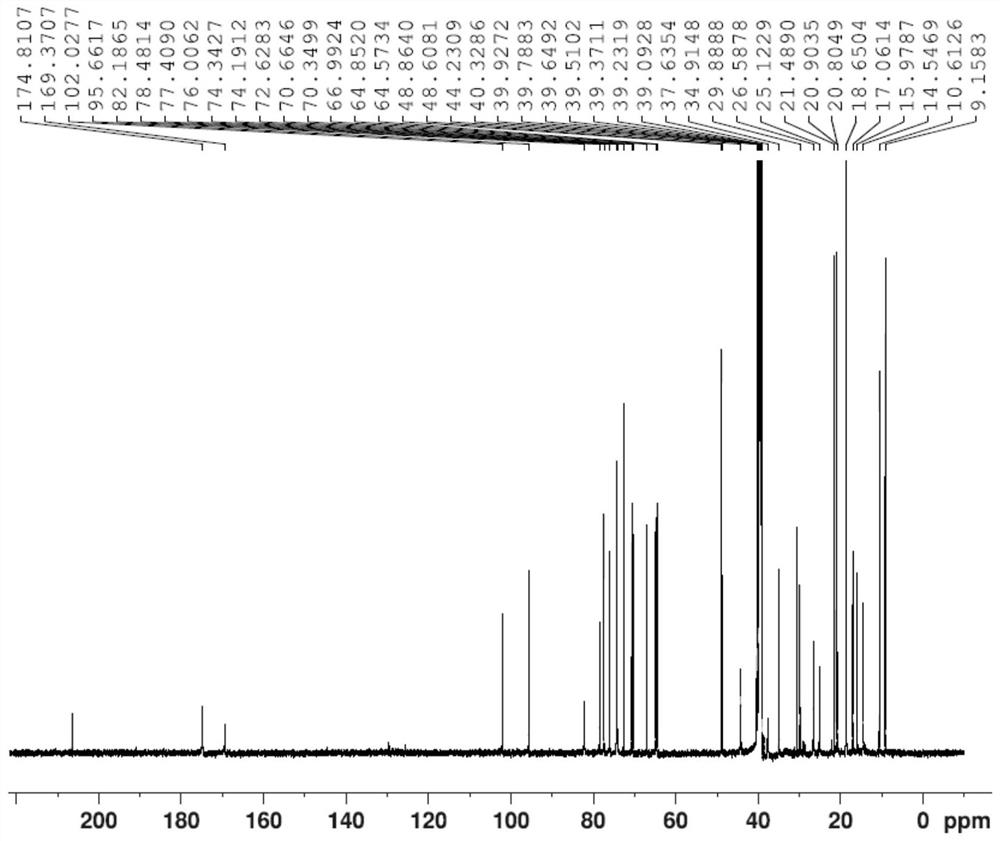
A kind of synthesis method of azithromycin rearrangement impurity lactam
A technique for the synthesis of azithromycin, applied in the field of chemistry, can solve the problems of only 55% yield, only 63%, complex step-by-step operation, etc., and achieve the effects of easy distillation, high solubility, and simple post-reaction treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 (2R, 3S, 4R, 5R, 8R, 10R, 11R, 12S, 13S, 14R)-11-[[(3,4,6)-trideoxy-3-(dimethylamino)-β- D-xyl-hexapyranosyl]oxy]-2-ethyl-3,4,10-trihydroxy-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl base-α-L-nucleo-hexapyranosyl)oxy]-3,5,8,10,12,14-hexamethyl-1-oxa-6-azacyclopentadecane-7,15 - Synthesis of diketones
[0032] (3R,4R,5S,6R,9R,10S,11S,12R,13R,15R,Z)-12-[[3,4,6-trideoxy-3-(dimethylamino)-β-D- Wood-hexyranosyl]oxy]-6-ethyl-4,5-dihydroxy-10-[(2,6-dideoxy-3-C-methyl-3-O-methyl-a-L- Nucleo-hexapyranosyl)oxy]-3,5,9,11,13,15-hexamethyl-7,16-dioxo-2-azabicyclo[11.2.1]hexadecane-1- Add 20g of en-8-one (compound II, 26mmol) and 100ml of dichloromethane into a three-neck flask, control the temperature at 20-25°C, add 1.72g (28.6mmol) of glacial acetic acid, and 3.95g (28.6mmol) of potassium carbonate Dissolve in 20ml of water, stir for 2-3 hours, add 20ml of water to wash, separate layers, take the organic layer and distill under reduced pressure to obtain 18.6g of white s...
Embodiment 2
[0034] Example 2 (2R, 3S, 4R, 5R, 8R, 10R, 11R, 12S, 13S, 14R)-11-[[(3,4,6)-trideoxy-3-(dimethylamino)-β- D-xyl-hexapyranosyl]oxy]-2-ethyl-3,4,10-trihydroxy-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl base-α-L-nucleo-hexapyranosyl)oxy]-3,5,8,10,12,14-hexamethyl-1-oxa-6-azacyclopentadecane-7,15 - Synthesis of diketones
[0035](3R,4R,5S,6R,9R,10S,11S,12R,13R,15R,Z)-12-[[3,4,6-trideoxy-3-(dimethylamino)-β-D- Wood-hexyranosyl]oxy]-6-ethyl-4,5-dihydroxy-10-[(2,6-dideoxy-3-C-methyl-3-O-methyl-a-L- Nucleo-hexapyranosyl)oxy]-3,5,9,11,13,15-hexamethyl-7,16-dioxo-2-azabicyclo[11.2.1]hexadecane-1- Add 20g of en-8-one (compound II, 26mmol), 50ml of dichloromethane into a three-necked flask, control the temperature at 20-25°C, add 1.72g (28.6mmol) of glacial acetic acid, 3.95g (28.6mmol) of potassium carbonate Dissolve in 20ml of water, stir for 2-3 hours, add 20ml of water to wash, separate layers, take the organic layer and distill under reduced pressure to obtain 17g of white solid, yie...
Embodiment 3
[0037] Example 3 (2R, 3S, 4R, 5R, 8R, 10R, 11R, 12S, 13S, 14R)-11-[[(3,4,6)-trideoxy-3-(dimethylamino)-β- D-xyl-hexapyranosyl]oxy]-2-ethyl-3,4,10-trihydroxy-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl base-α-L-nucleo-hexapyranosyl)oxy]-3,5,8,10,12,14-hexamethyl-1-oxa-6-azacyclopentadecane-7,15 - Synthesis of diketones
[0038] (3R,4R,5S,6R,9R,10S,11S,12R,13R,15R,Z)-12-[[3,4,6-trideoxy-3-(dimethylamino)-β-D- Wood-hexyranosyl]oxy]-6-ethyl-4,5-dihydroxy-10-[(2,6-dideoxy-3-C-methyl-3-O-methyl-a-L- Nucleo-hexapyranosyl)oxy]-3,5,9,11,13,15-hexamethyl-7,16-dioxo-2-azabicyclo[11.2.1]hexadecane-1- Add 20g of en-8-one (compound II, 26mmol) and 100ml of dichloromethane into a three-neck flask, control the temperature at 40-45°C, add 1.72g (28.6mmol) of glacial acetic acid, and 3.95g (28.6mmol) of potassium carbonate Dissolve in 20ml of water, stir for 2-3 hours, add 20ml of water to wash, separate layers, take the organic layer and distill under reduced pressure to obtain 16.2g of white s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com