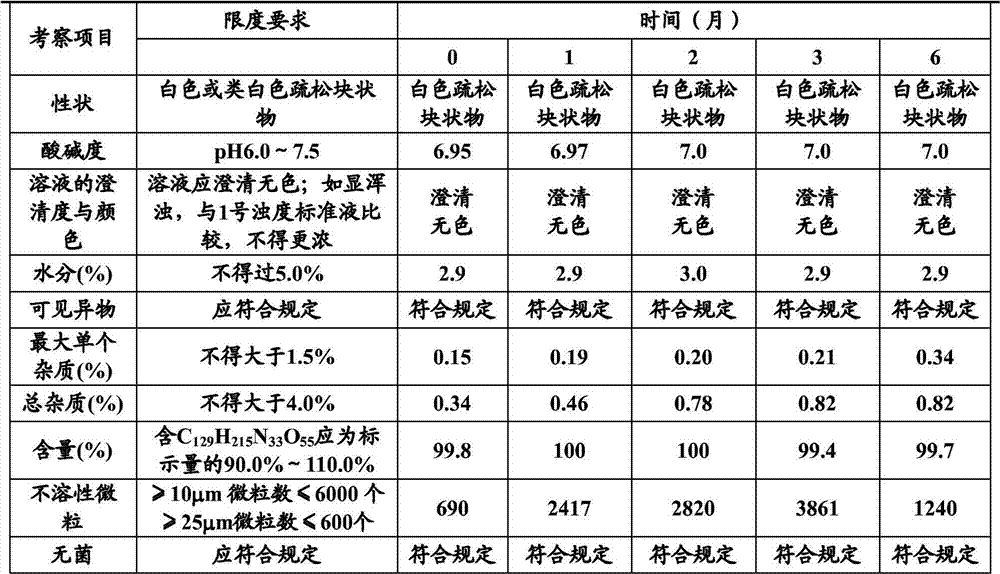
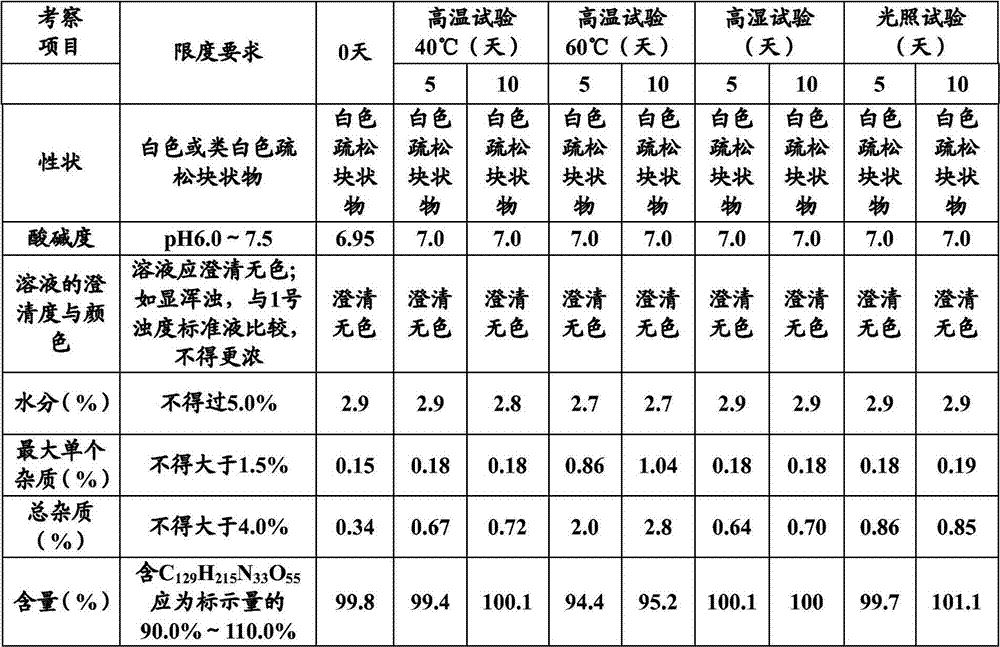
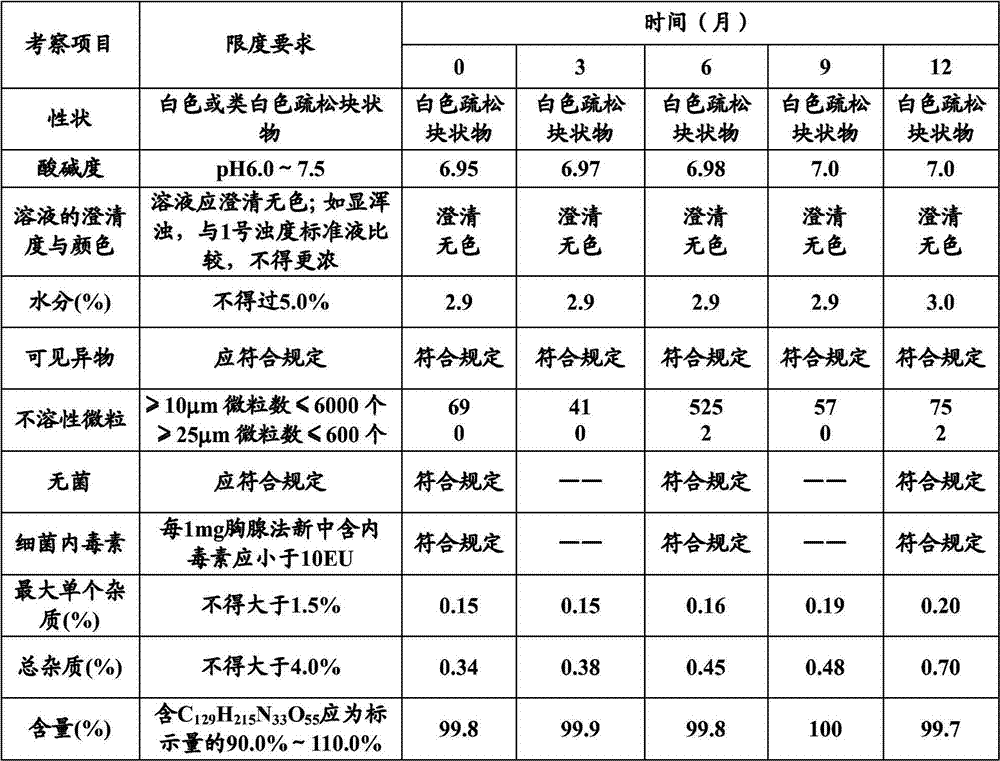
Stable thymalfasin preparation and preparation method thereof
A thymus method and preparation technology, which is applied in the field of medicine, can solve the problems of poor appearance of finished preparations, inconvenient clinical use, substandard quality, etc., and achieve the effects of avoiding adverse reactions, facilitating drug distribution, and increasing safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A kind of preparation method of stable thymus method new preparation, it comprises the steps:
[0046] (1) Add 0.1g of thymofasin and 10g of mannitol into about 800ml of water at 15°C to 25°C, stir to dissolve, measure the pH value, use a phosphate buffer solution with a pH of 7.4 (made of disodium hydrogen phosphate aqueous solution and phosphoric acid dihydrogen sodium aqueous solution) adjust the pH value to 6.8-7.2, make up the water volume to 1L, and control the solution temperature at 15°C-25°C during the whole process;
[0047] (2), add 0.5 g of medicinal activated carbon to the solution after step (1), adsorb at 15°C to 25°C for 15 minutes, decarbonize and filter, and then use a 0.22 μm microporous membrane to perform sterilizing filtration;
[0048] (3), the solution filtered through step (2) is sub-packed in 1000 cillin bottles, the loading capacity is 1ml, half stoppered, sampling is measured, and the content of thymus method is 0.1mg / ml, and the pH of the so...
Embodiment 2
[0052] A kind of preparation method of stable thymus method new preparation, it comprises the steps:
[0053] (1) Add 1.6g of thymofasin and 10g of mannitol into about 800ml of water at 15°C to 25°C, stir to dissolve, measure the pH value, use a phosphate buffer solution with a pH of 7.4 (made of disodium hydrogen phosphate aqueous solution and phosphoric acid dihydrogen sodium aqueous solution) adjust the pH to 6.8-7.2, add water to 1L, and control the solution temperature at 15°C-25°C during the whole process;
[0054] (2) Add 0.5 g of medicinal activated carbon, adsorb at 15°C to 25°C for 15 minutes, decarbonize and filter, and then use a 0.22 μm microporous membrane to perform sterilizing filtration;
[0055] (3), the solution filtered through step (2) is sub-packed in 1000 cillin bottles, the loading capacity is 1ml, half stoppered, sampling is measured, and the content of thymosin is 1.6mg / ml, and the pH of the solution is 6.94;
[0056] (4) Use a vacuum freeze dryer to...
Embodiment 3
[0059] A kind of preparation method of stable thymus method new preparation, it comprises the steps:
[0060] (1) Add 2g of thymosin and 20g of mannitol into about 800ml of water at 15°C to 25°C, stir and dissolve, measure the pH value, and use a phosphate buffer solution with a pH of 7.4 (made of disodium hydrogen phosphate aqueous solution and diphosphate Hydrogen-sodium aqueous solution) adjust the pH to 6.8-7.2, make up the water volume to 1L, and control the solution temperature at 15°C-25°C during the whole process;
[0061] (2) Add 0.5 g of medicinal activated carbon, adsorb at 15°C to 25°C for 15 minutes, decarbonize and filter, and then use a 0.22 μm microporous membrane to perform sterilizing filtration;
[0062] (3), the solution filtered through step (2) is sub-packed in 1000 cillin bottles, the loading capacity is 1ml, half stoppered, and sampling is determined, and the content of thymosin is 2mg / ml, and the pH is 6.95;
[0063] (4) Use a vacuum freeze dryer to p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com