Doped and surface coating co-modified anode material for lithium ion battery and preparation method thereof
A lithium-ion battery and surface coating technology, applied in the field of materials, can solve the problems of unresearched electrochemical performance, doping and modification of positive electrode materials, electrochemical performance research, etc., achieve good cycle performance, simple operation, and easy realization Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] (1) Weigh 39.19g Li respectively 2 CO 3 , 82.01g Co 3 o 4 and 0.41g MgO, mixed by ball milling for 4 hours, sintered at 1050°C for 12 hours, naturally cooled to room temperature, crushed and sieved to prepare the doped lithium cobaltate cathode material matrix;
[0091] (2) Take 4.805g Co(NO 3 ) 2 ·6H 2 Dissolve O in 15mL deionized water to make solution A;
[0092] (3) Take 1.455g (NH 4 ) 2 HPO 4 Dissolve in 15mL deionized water to make solution B;
[0093] (4) Add liquid B dropwise to liquid A at a rate of 0.5mL / min and keep stirring to form a stable sol C, and add ammonia water to adjust the pH of sol C to 8;
[0094] (5) Take 100g of the doped lithium cobalt oxide cathode material matrix prepared in step (1), and evenly disperse it into the C solution prepared in (4), stir once every 5 minutes, and dry it in an oven at 120°C after 1 hour. After passing through a 200-mesh sieve, the doped and surface-coated co-modified lithium cobaltate precursor was obtai...
Embodiment 2
[0098] (1) Weigh 38.05gLi respectively 2 CO 3 and 82.01gCo 3 o 4 , mixed ball mill, obliquely mixed for 2 hours, sintered at 1000°C for 10 hours, cooled naturally to room temperature, crushed and sieved to obtain LiCoO 2 ;
[0099] (2) Weigh 2g TiO respectively 2 and 100g of LiCoO produced in step (1)2 , after ball milling for 1 hour, sintering at 1030°C for 10 hours, naturally cooling to room temperature, crushing and sieving, to prepare the doped lithium cobaltate cathode material matrix;
[0100] (3) Weigh 4.118g Al(NO 3 ) 3 9H 2 Dissolve O in 15mL deionized water to make solution A;
[0101] (4) Another 2.166g (NH 4 ) 2 HPO 4 Dissolve in 15mL deionized water to make solution B;
[0102] (5) Add liquid B dropwise to liquid A at a rate of 0.5mL / min and keep stirring to form a stable sol C, and add ammonia water dropwise to adjust the pH of sol C to 8;
[0103] (6) Take 100g of the doped lithium cobalt oxide cathode material matrix prepared in step (2), and evenl...
Embodiment 3
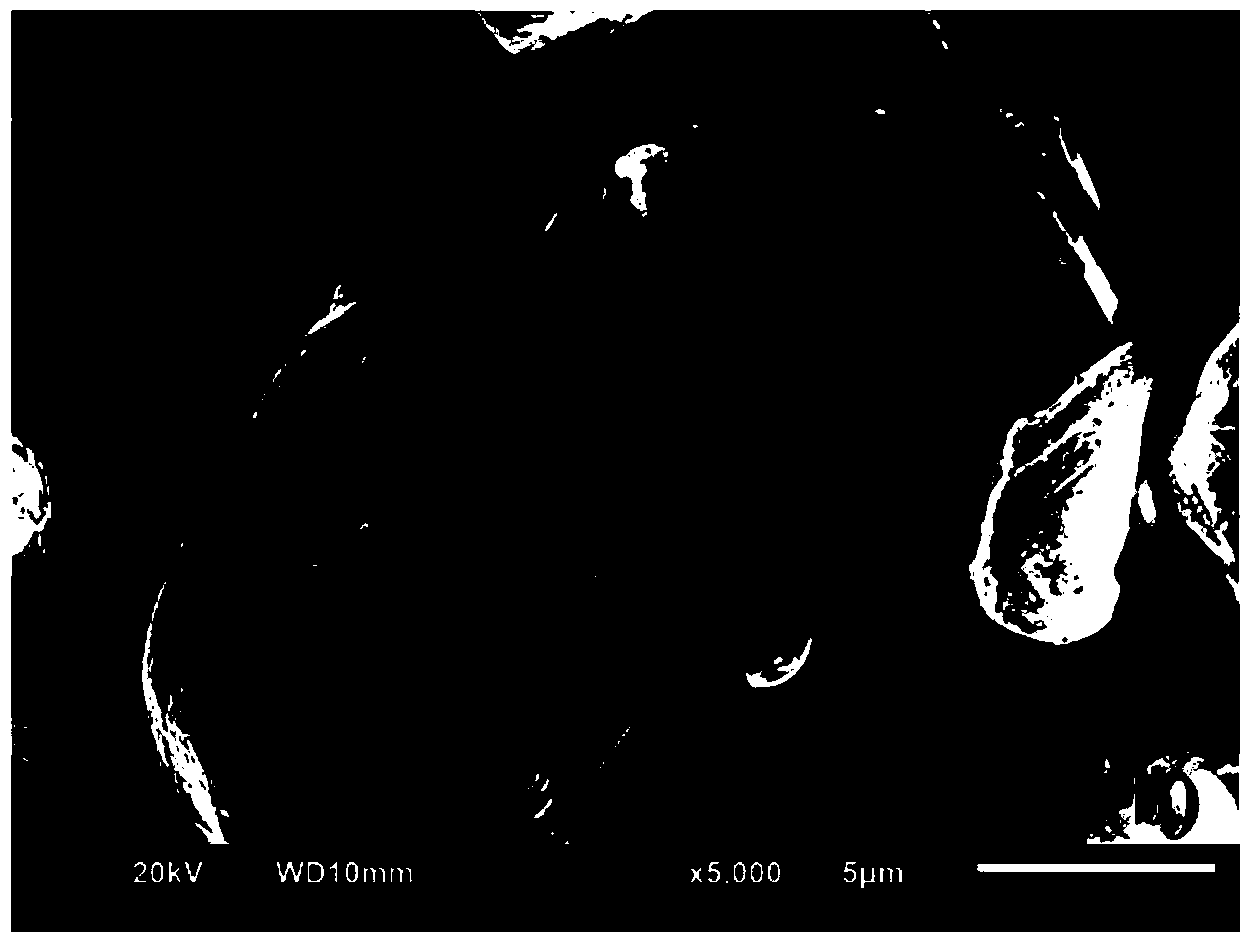
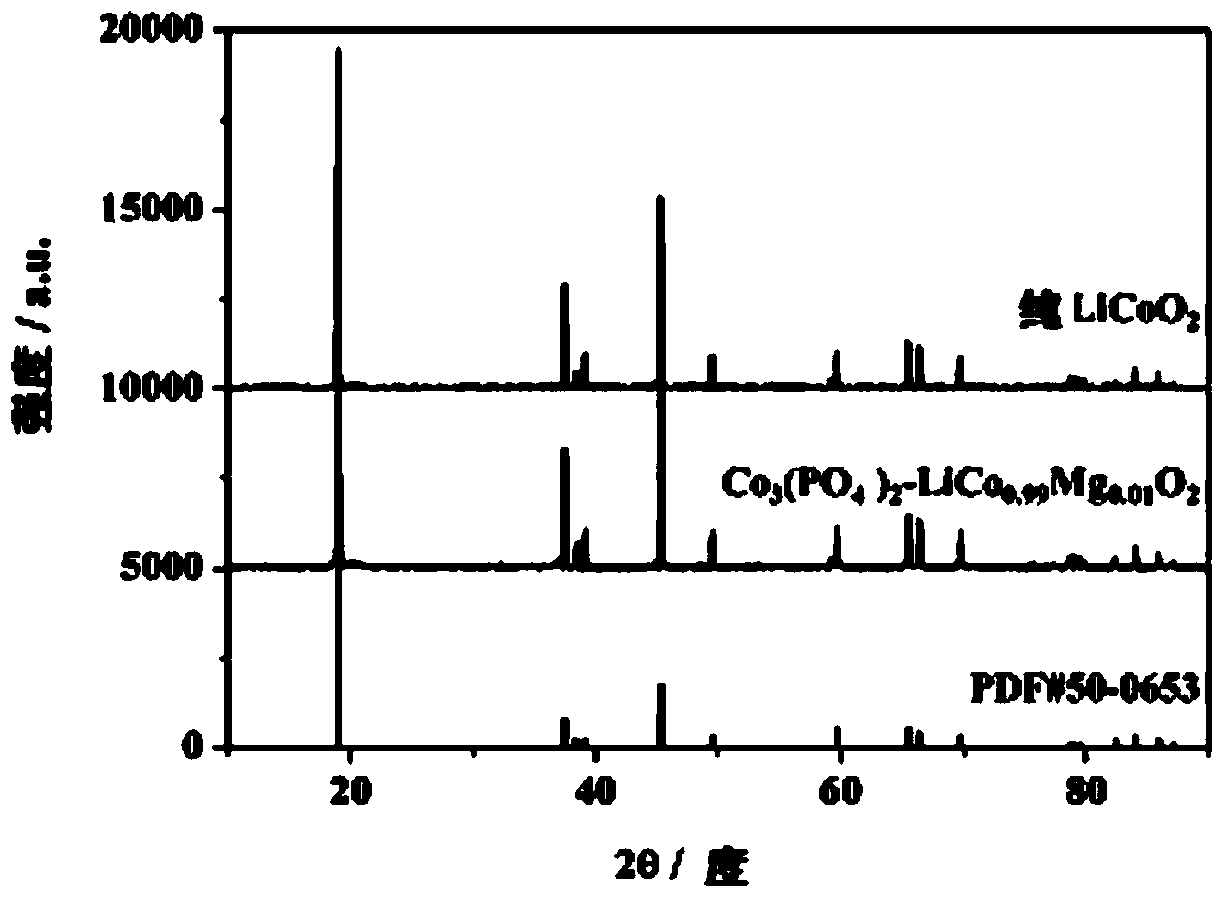
[0106] The method of this embodiment is the same as that of Example 1, except that the solvent used in step (2) is a 90% aqueous solution of ethanol, and the obtained sample of Example 3 is Co 3 (PO 4 ) 2 -LiCo 0.99 Mg 0.01 o 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com