Thymalfasin crystal and pharmaceutical composition thereof
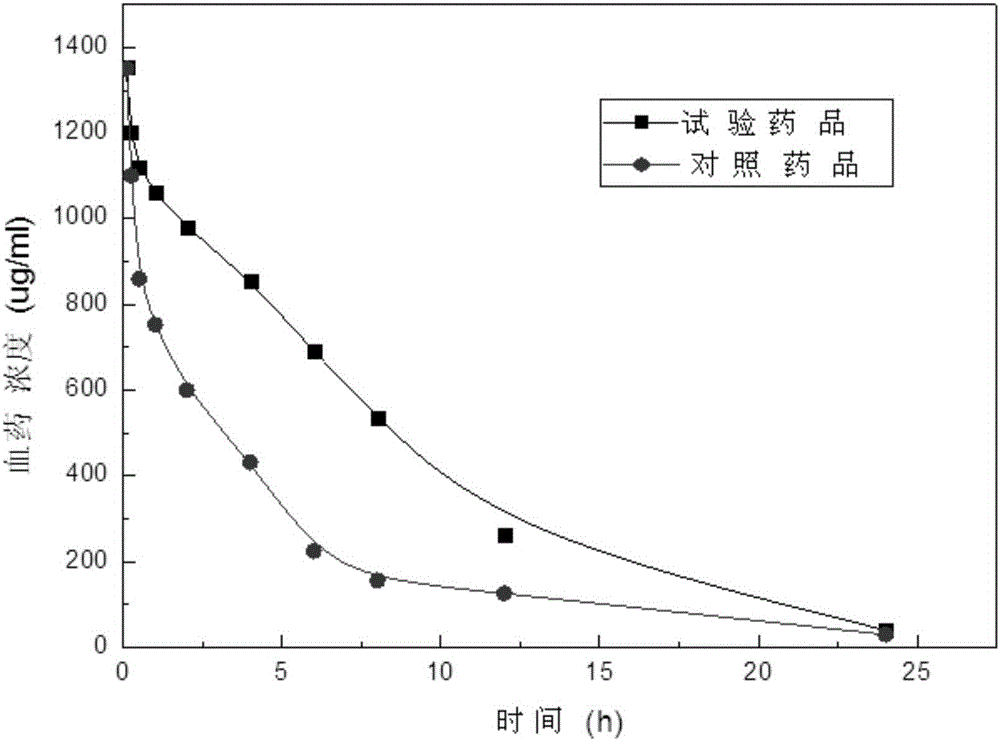
A thymus method and composition technology, applied in the field of medicine, can solve the problems of easy production of impurities, unqualified quality, low bioavailability, etc., achieve good stability and solubility, ease the blood drug concentration curve, and bioavailability Improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation method of the new crystal of embodiment 1 thymus method:
[0040] (1) thymus fasin is dissolved in the aqueous solution mixed with methylene chloride and ethyl bromide, the volume ratio of methylene chloride and ethyl bromide is 2:11, the total mass fraction of methylene chloride and ethyl bromide is 50%, the solution The pH value is 7, and the temperature is 25°C;
[0041] (2) Stir the above solution to cool down, the stirring rate is 5rmp, the stirring time is 45min, cool down to -40°C, the cooling rate is 1.5°C / h, and stand for 10h;
[0042] (3) After washing, filtering, and drying, the moisture content after drying is 3.9%, and the thymus method new crystals are obtained.
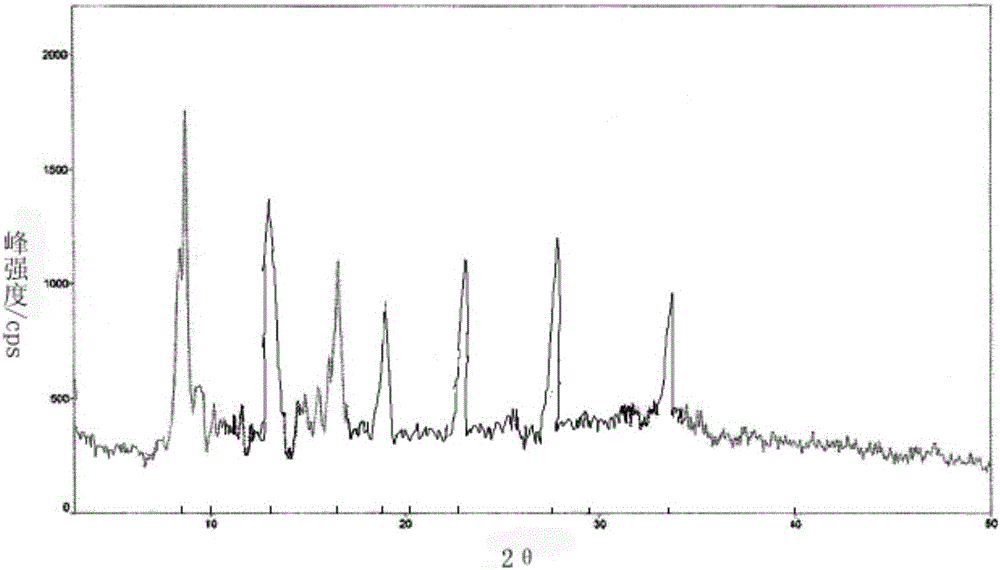
[0043] The X-ray powder diffraction spectrum that the obtained thymus method uses Cu-Kα ray measurement to obtain is as follows figure 1 shown.
Embodiment 2
[0044] The preparation method of the new crystal of embodiment 2 thymus method
[0045] (1) Thymofasin is dissolved in the aqueous solution mixed with bromoethane, the volume ratio of dichloromethane to bromoethane is 2:13, the total mass fraction of dichloromethane and bromoethane is 52%, the pH value of the solution 7. The temperature is 25°C;
[0046] (2) Stir the above solution to cool down, the stirring rate is 4rmp, the stirring time is 40min, cool down to -20°C, the cooling rate is 1.0°C / h, and stand for 7h;
[0047] (3) After washing, filtering and drying, the moisture content after drying is 3.0%, and the thymus method new crystals are obtained.
[0048] The X-ray powder diffraction spectrum obtained by measuring the thymus method using Cu-Kα rays is basically consistent with that of Example 1.
Embodiment 3
[0049] The preparation method of the new crystal of embodiment 3 thymus method
[0050] (1) thymus fasin is dissolved in the aqueous solution mixed with bromoethane, the volume ratio of dichloromethane to bromoethane is 5:11, the total mass fraction of dichloromethane and bromoethane is 60%, the pH value of the solution 6. The temperature is 15°C;
[0051] (2) Stir the above solution to cool down, the stirring rate is 2rmp, the stirring time is 30min, cool down to -40°C, the cooling rate is 0.5°C / h, and stand for 5h;
[0052] (3) After washing, filtering and drying, the moisture content after drying is 4.9%, and the thymus method new crystal is obtained.
[0053] The X-ray powder diffraction spectrum obtained by measuring the thymus method using Cu-Kα rays is basically consistent with that of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com