Freeze-drying process of thymalfasin for injection
A thymosin and injection technology, which is applied in the freeze-drying process of thymosin for injection, can solve the problems of increasing the impurity content of the finished product and the long time, and achieve the effects of easy use and good resolubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: A new freeze-drying process of thymus for injection
[0041] Described freeze-drying process comprises the following steps successively:
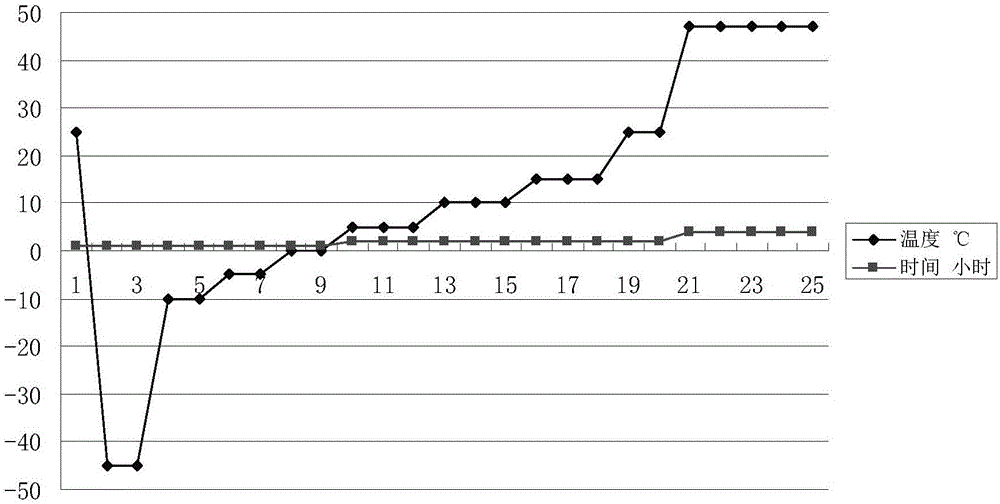
[0042] (1) Pre-freezing: transfer the new thymus method product to the freezer of the freeze dryer, and the new thymus method product conducts the cold through the plate in the freeze dryer, adjusts the temperature of the inner plate layer of the freeze dryer, and puts the plate layer The temperature of the new product of the upper thymus method is reduced to -45°C, and it is kept warm for 0.5 hours; pressure in the cavity;
[0043](2) One-time sublimation drying, adopting seven-stage stepped heating: a. Directly heat the refrigerant in the slab to -10°C at a heating rate of 1.5°C / min and keep it warm for 1 hour; when the freezer cavity When the vacuum degree in the body reaches 15 Pa, the pulsating air infiltration is automatically carried out in the cavity of the freezer, and the infiltration is stopped when the vac...
Embodiment 2
[0053] Embodiment 2: A kind of new freeze-drying process of thymus method for injection
[0054] Described freeze-drying process comprises the following steps successively:
[0055] (1) Pre-freezing: transfer the new thymus method product to the freezer of the freeze dryer, and the new thymus method product conducts the cold through the plate in the freeze dryer, adjusts the temperature of the inner plate layer of the freeze dryer, and puts the plate layer The temperature of the new product of the upper thymus method is reduced to -42°C, and it is kept warm for 0.5 hours; after the heat preservation is completed, the temperature of the cold trap of the lyophilizer is dropped to -58°C, and it is kept warm for 0.5 hours, then vacuuming is started, and the freezer is lowered pressure in the cavity;
[0056] (2) One-time sublimation drying, adopting seven-stage stepped heating: a. Directly heat the refrigerant in the slab to -11°C, with a heating rate of 1.5°C / min, and keep warm ...
Embodiment 3
[0066] Embodiment 3: A kind of new freeze-drying process of thymus method for injection
[0067] Described freeze-drying process comprises the following steps successively:
[0068] (1) Pre-freezing: transfer the new thymus method product to the freezer of the freeze dryer, and the new thymus method product conducts the cold through the plate in the freeze dryer, adjusts the temperature of the inner plate layer of the freeze dryer, and puts the plate layer The temperature of the new product of the upper thymus method is reduced to -40°C, and it is incubated for 1 hour; after the end of the incubation, the temperature of the cold trap of the lyophilizer is reduced to -50°C, it is incubated for 0.5 hours and vacuumized, and the freezer is lowered. pressure in the cavity;
[0069] (2) One-time sublimation drying, adopting seven-stage stepped heating: a. Directly heat the refrigerant in the slab to -9°C, the heating rate is 1.0°C / min, and keep warm for 1.5 hours; when the freezer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com