Compound in-situ gel long-acting injection for treating chronic hepatitis and preparation method thereof
A gel injection and in-situ gel technology, applied in the field of medicine, can solve the problems of patients forgetting to take medicine, affecting curative effect, and long treatment cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
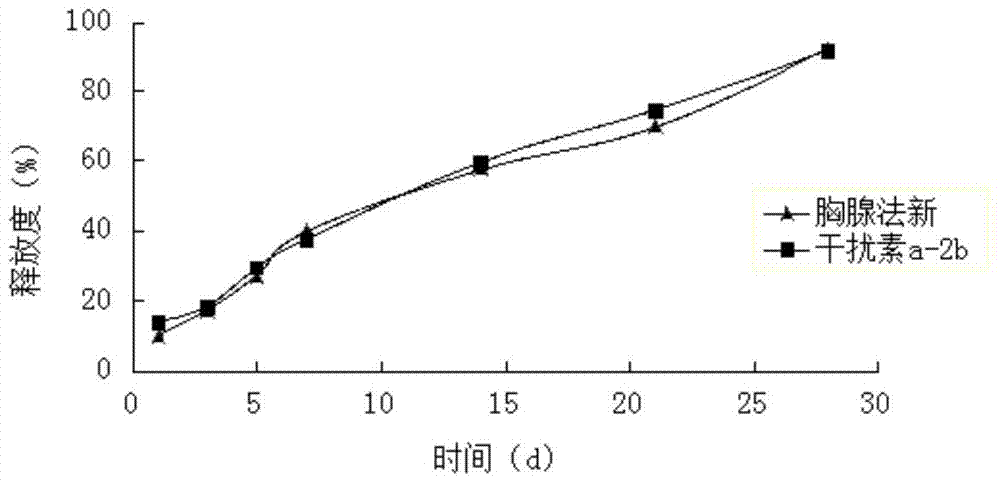
[0064] Weigh 15 mg of interferon a-2b and dissolve it in sterile water for injection, add 35 mg of zinc hydroxide that has been micronized and sterilized, and vortex and mix for 10 minutes to form 1 g of zinc salt interferon a-2b solution.
[0065] Weigh 2.6g PLGA (weight-average molecular weight 10000, intrinsic viscosity 0.10dl / g-0.20dl / g, LA:GA=75:25), 0.9g poloxamer 407 and 18g NMP, mix well, and prepare a polymer Then add 0.30g of Thymusfaxin raw material drug, ultrasonically dissolve, add 1g of zinc salt interferon a-2b solution after complete dissolution, heat at constant temperature and stir at high speed (temperature 30°C, 10000rpm) for 5min to form drug-loaded particles The sol system is sterilized by electron beams to obtain the compound long-acting in-situ gel injection. The drug loading amount of interferon was detected by HPLC to be 0.37%, and the encapsulation efficiency was 95%. The drug loading of thymus method was 7.18%, and the encapsulation rate was 92%. ...
Embodiment 2
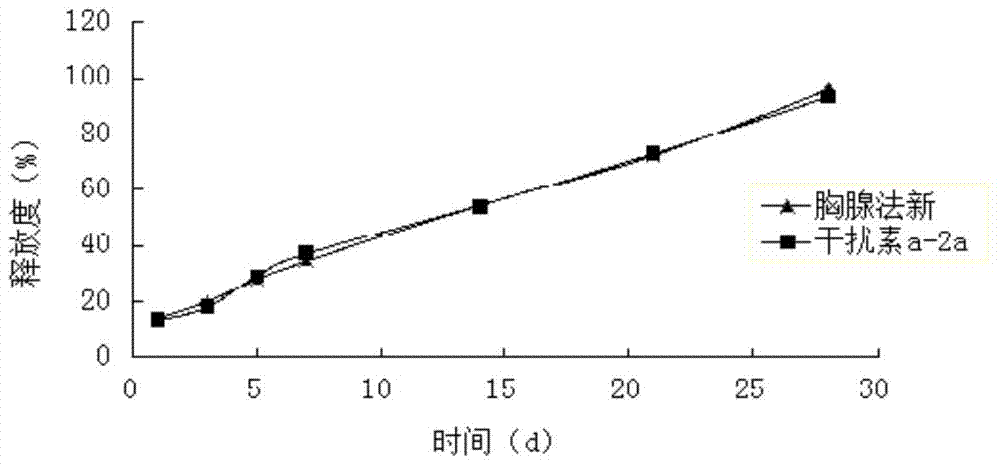
[0069] Weigh 20 mg of interferon a-2a and dissolve it in sterile water for injection, add 55 mg of zinc chloride after micronization and sterilization, and vortex and mix for 10 minutes to form 1 g of zinc salt interferon a-2a solution.
[0070] Weigh 4.0g PLGA (weight average molecular weight 15000, intrinsic viscosity 0.10dl / g-0.25dl / g, LA:GA=65:35), 2.0g methylcellulose and 45g ethyl lactate, mix well, and prepare Polymer solution, then add 0.80g of Thymofaxin API, ultrasonically dissolve, add 1g of zinc salt interferon a-2a solution after complete dissolution, heat at constant temperature and stir at high speed (temperature 30°C, 10000rpm) for 7min, to form a carrier containing particles The drug sol system is sterilized by electron beams to obtain the compound long-acting in-situ gel injection. The drug loading amount of interferon was detected by HPLC to be 0.33%, and the encapsulation efficiency was 97%. The drug loading capacity of thymus method was 12.53%, and the en...
Embodiment 3
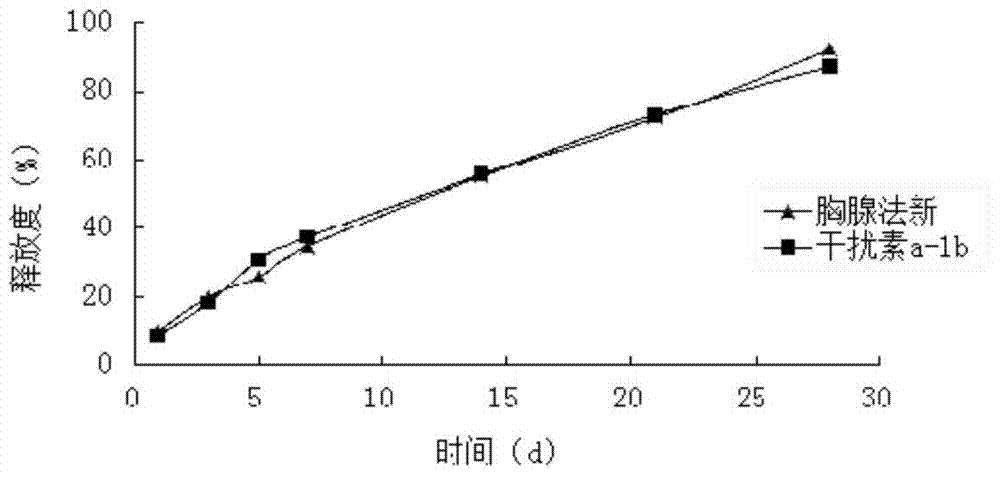
[0074] Weigh 25 mg of interferon a-1b and dissolve it in sterile water for injection, add 55 mg of magnesium hydroxide that has been micronized and sterilized, and vortex and mix for 10 minutes to form a solution of 1 g of magnesium salt interferon a-1b.
[0075] Weigh 3.75g PLA (weight-average molecular weight 15000, intrinsic viscosity 0.10dl / g-0.25dl / g), 3.75g Carbomer 934 and 35g acetone, mix well, and prepare polymer solution, then add new thymus method raw material 0.75g of drug, ultrasonically dissolved, after complete dissolution, add 1g of magnesium salt interferon a-1b solution, heat at constant temperature and high-speed stirring (temperature 30°C, 10000rpm) for 5min, to form a drug-loaded sol system containing particles, and sterilize with electron beams , to obtain compound long-acting in situ gel injection. The drug loading amount of interferon was detected by HPLC to be 0.28%, and the encapsulation efficiency was 98%. The drug loading of thymus method was 12.44...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com