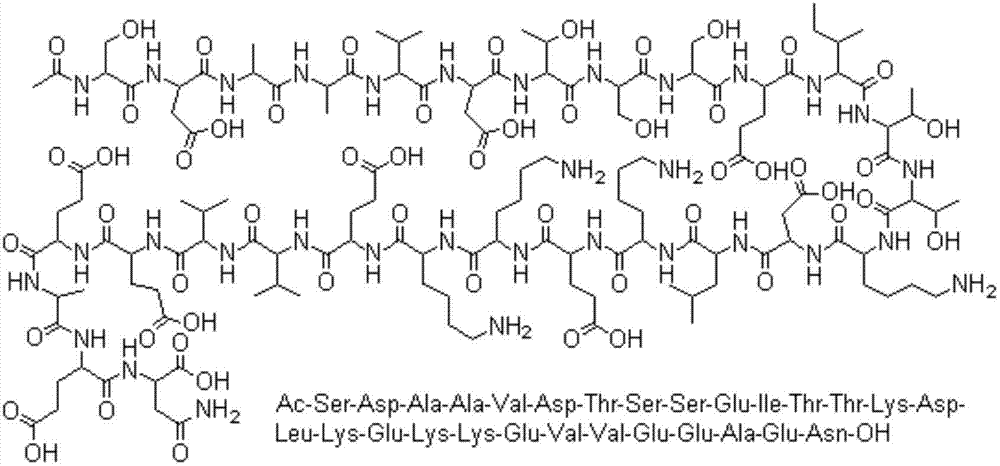
Thymalfasin-containing freeze-dried preparation
A new technology of freeze-dried preparation and thymus method, which is applied in the field of medicine, can solve the problems of increased impurity content and poor stability, and achieves the effect of improving the bacteriostatic effect, shortening the freeze-drying period, and having a significant curative effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] prescription:
[0030]
[0031] Preparation:
[0032] 1. Take 50% of the prescribed amount of water for injection, add tert-butanol, mannitol, and sodium acetate in sequence and stir to dissolve.
[0033] 2. Take the prescribed amount of Thymus Faxin and add it to the above-mentioned solution, stir to dissolve completely.
[0034] 3. Adjust the pH value of the solution to 5.5-6.5 with 0.5M acetic acid or sodium acetate.
[0035] 4. Dilute the above solution to 1000ml with water for injection.
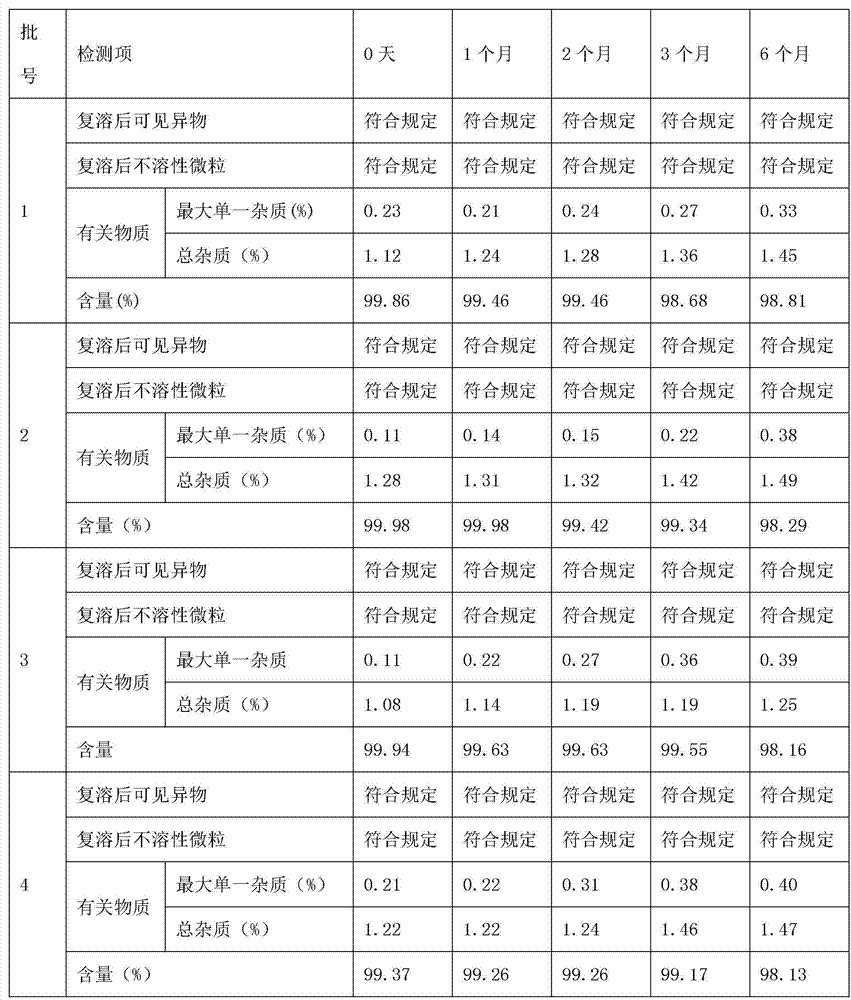
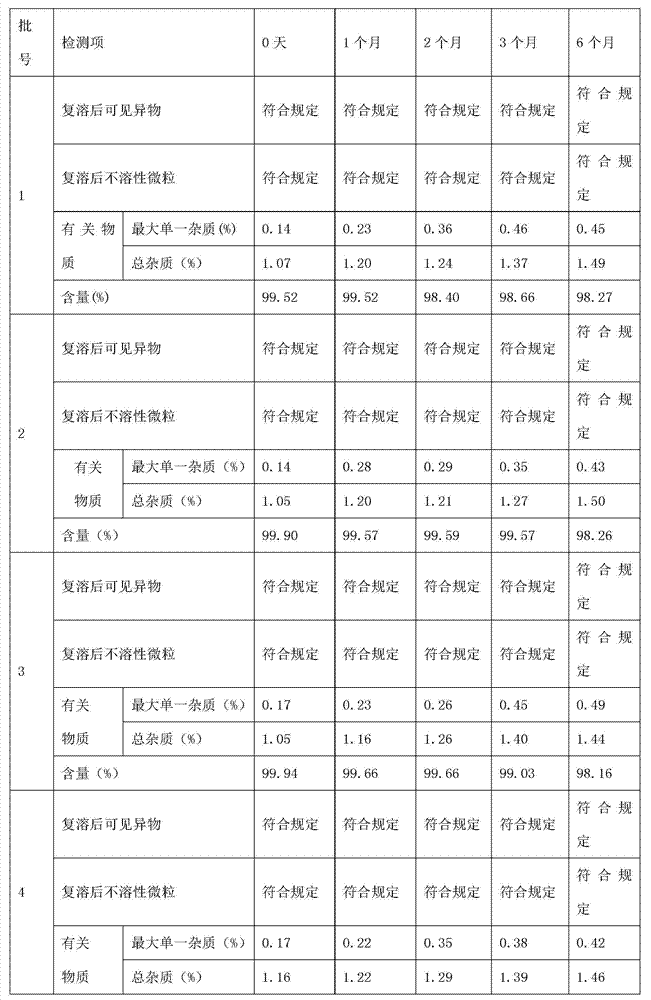
[0036] 5. Use a tangential flow ultrafiltration system with a molecular weight cut-off of 5,000 Daltons to filter the above-mentioned medicinal solution to remove heat sources and possible microorganisms, and detect the content of thymosin in it.
[0037] 6. Divide the liquid medicine obtained above into 1ml colorless medium borosilicate glass vials, and half stoppered.
[0038] 7. Put the control bottle containing the above-mentioned liquid medicine into the cold trap of ...
Embodiment 2
[0041] prescription:
[0042]
[0043] Preparation:
[0044] 1. Take 50% of the prescribed amount of water for injection, add tert-butanol, mannitol, and sodium acetate in sequence and stir to dissolve.
[0045] 2. Take the prescribed amount of Thymus Faxin and add it to the above-mentioned solution, stir to dissolve completely.
[0046] 3. Adjust the pH value of the solution to 5.5-6.5 with 0.5M acetic acid or sodium acetate.
[0047] 4. Dilute the above solution to 1000ml with water for injection.
[0048] 5. Use a tangential flow ultrafiltration system with a molecular weight cut-off of 5,000 Daltons to filter the above-mentioned medicinal solution to remove heat sources and possible microorganisms, and detect the content of thymosin in it.
[0049] 6. Divide the liquid medicine obtained above into 1ml colorless medium borosilicate glass vials, and half stoppered.
[0050] 7. Put the control bottle containing the above-mentioned liquid medicine into the cold trap of ...
Embodiment 3
[0053] prescription:
[0054]
[0055] Preparation:
[0056] 1. Take 50% of the prescribed amount of water for injection, add tert-butanol, mannitol, and sodium acetate in sequence and stir to dissolve.
[0057] 2. Take the prescribed amount of Thymus Faxin and add it to the above-mentioned solution, stir to dissolve completely.
[0058] 3. Adjust the pH value of the solution to 5.5-6.5 with 0.5M acetic acid or sodium acetate.
[0059] 4. Dilute the above solution to 1000ml with water for injection.
[0060] 5. Use a tangential flow ultrafiltration system with a molecular weight cut-off of 5,000 Daltons to filter the above-mentioned medicinal solution to remove heat sources and possible microorganisms, and detect the content of thymosin in it.
[0061] 6. Divide the liquid medicine obtained above into 1ml colorless medium borosilicate glass vials, and half stoppered.
[0062] 7. Put the control bottle containing the above-mentioned liquid medicine into the cold trap of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com