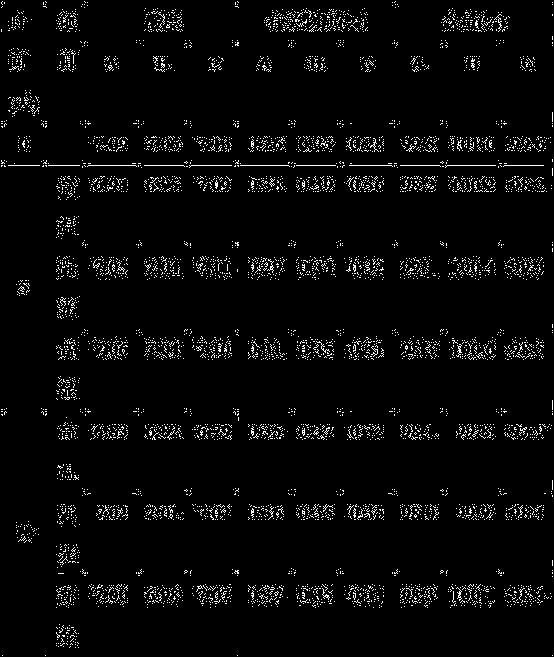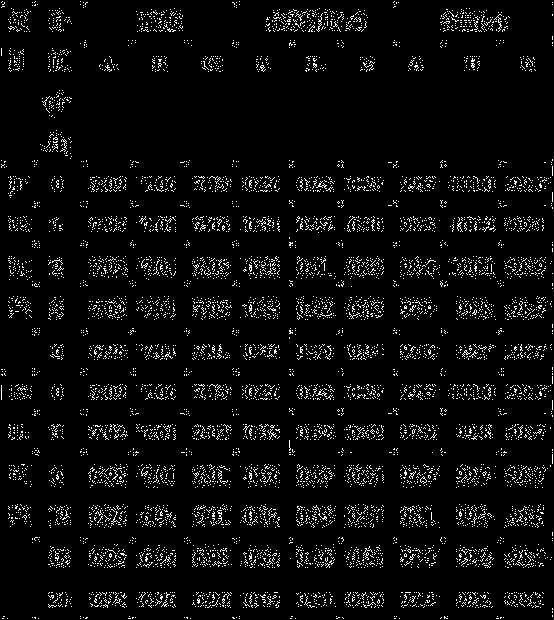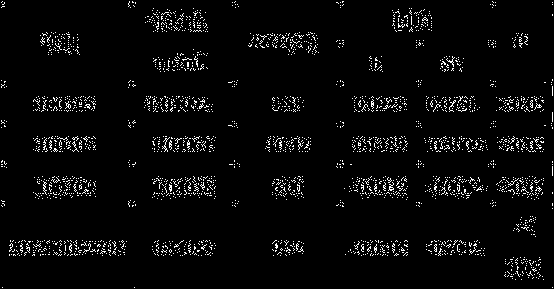Thymalfasin-containing drug composition and preparation thereof
A technology of thymus method and composition, which is applied in the field of medicine, can solve problems such as platelet aggregation chain reaction and vascular endothelial cell damage, and achieve the effects of easy industrial production, convenient storage and transportation, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Under clean conditions, put 40g of mannitol into the liquid preparation device, add 700ml of water for injection and stir to dissolve, cool to room temperature, add 1.8g of disodium hydrogen phosphate, 0.2g of sodium dihydrogen phosphate, stir well, add 0.1% ( g / ml) with activated carbon for needles, stirred and adsorbed, and filtered to remove carbon; after the above preparation solution dropped to room temperature, add 1.5g thymus fasin, measure the pH value, and adjust the pH to 6.53 with sodium phosphate buffer (pH=7.2). , and then adjust the pH to 6.65 with tris-hydrochloric acid buffer solution (pH 7.7), add water for injection to the full amount, and filter through a 0.22 μm microporous membrane to obtain the Thymusfaxin filtrate. Dispense 3ml into ampoules, freeze-dry for 72 hours to make sterile freeze-dried powder injection.
Embodiment 2
[0065] Under clean conditions, put 45g of mannitol into the liquid preparation device, add 700ml of water for injection and stir to dissolve, cool to room temperature, add 1.9g of disodium hydrogen phosphate, 0.3g of sodium dihydrogen phosphate, stir well, add 0.1% ( g / ml) with activated carbon for needles, stirred and adsorbed, and filtered to remove carbon; after the above preparation solution dropped to room temperature, add 1.6g thymus fasin, measure the pH value, and adjust the pH to 6.55 with sodium phosphate buffer (pH=7.2). , and then adjust the pH to 6.7 with tris-hydrochloric acid buffer solution (pH 7.7), add water for injection to the full amount, and filter through a 0.22 μm microporous membrane to obtain the thymus method new filtrate. Dispense 3ml into ampoules, freeze-dry for 72 hours to make sterile freeze-dried powder injection.
Embodiment 3
[0067] Under clean conditions, put 50g of mannitol into the liquid dispensing device, add 700ml of water for injection and stir to dissolve, cool to room temperature, add 2.0g of disodium hydrogen phosphate, 0.4g of sodium dihydrogen phosphate, stir well, add 0.1% ( g / ml) with activated carbon for needles, stirred and adsorbed, and filtered to remove carbon; after the above preparation solution dropped to room temperature, add 1.7g thymusfasin, measure the pH value, and adjust the pH to 6.6 with sodium phosphate buffer (pH=7.2). , and then adjust the pH to 7.0 with tris-hydrochloric acid buffer solution (pH 7.7), add water for injection to the full amount, and filter through a 0.22 μm microporous membrane to obtain the thymus method new filtrate. Dispense 3ml into ampoules, freeze-dry for 72 hours to make sterile freeze-dried powder injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com