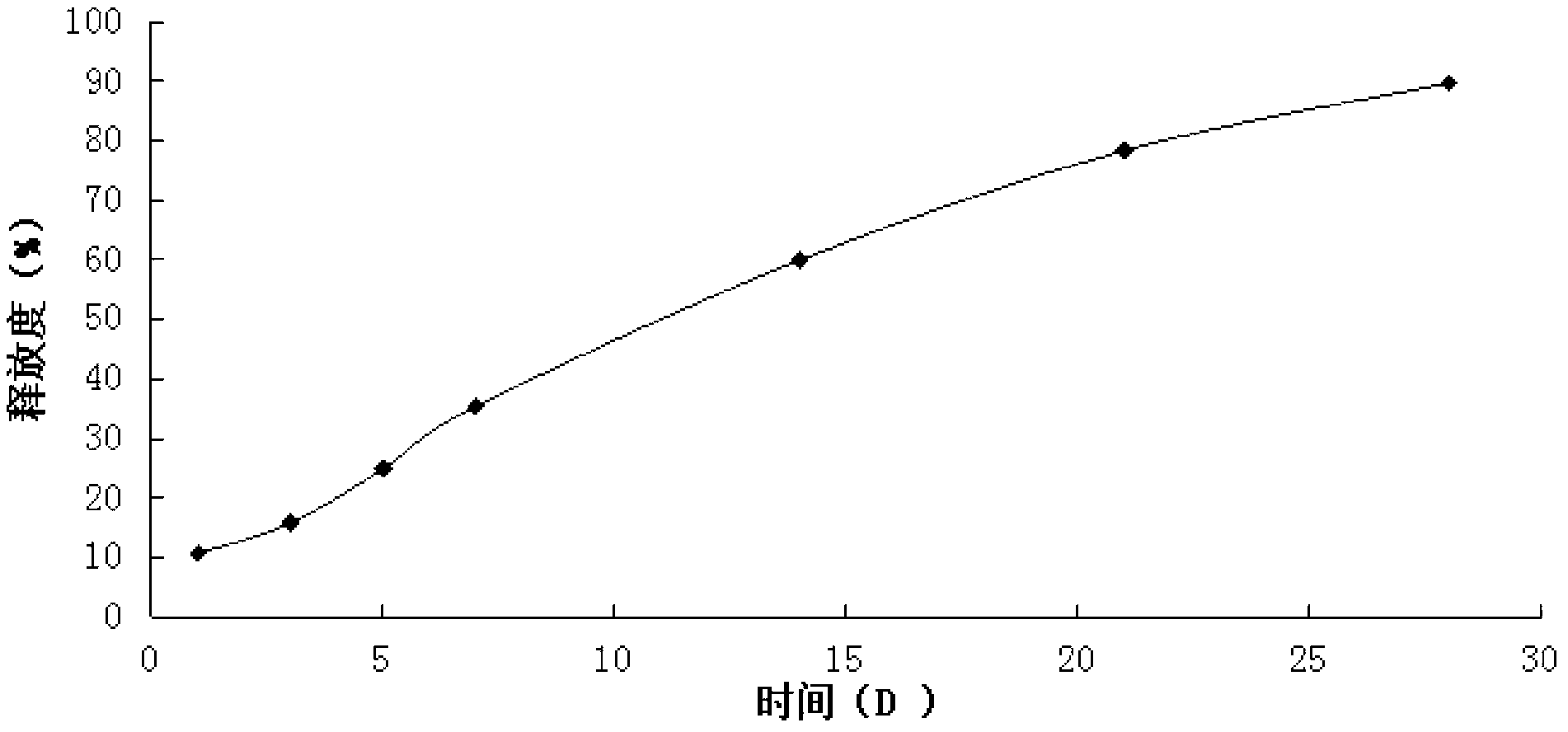
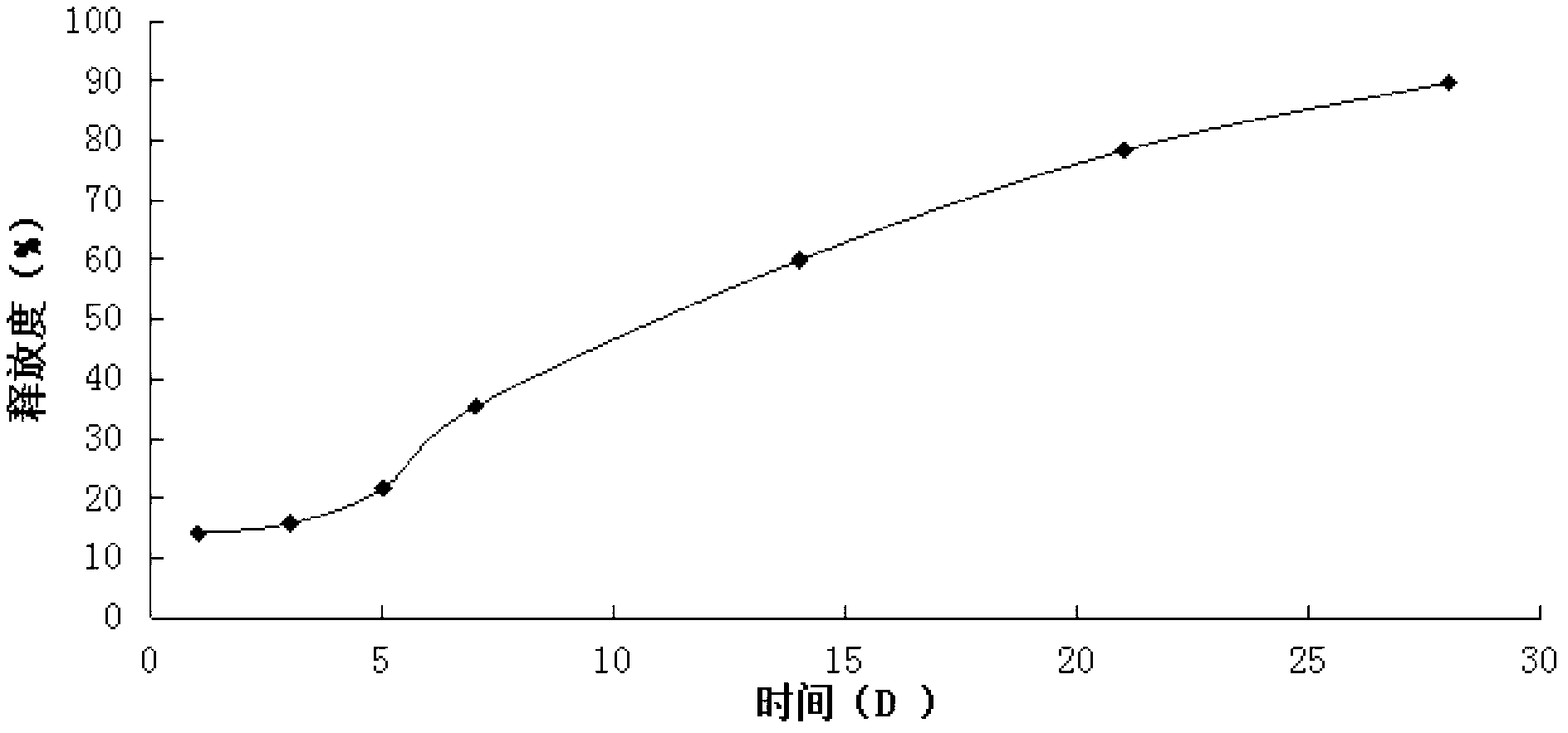
Thymalfasin in-situ gel preparation and preparation method thereof
A new technology of in situ gel and thymus method, applied in the directions of antiviral agents, pharmaceutical formulations, aerosol delivery, etc., can solve the problems of peak and valley phenomenon of blood drug concentration, short drug action time, adverse reactions, etc., to avoid The peak and trough of blood drug concentration, the clear structure of skin and connective tissue, the effect of alleviating pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Preparation of new in situ gel preparation of thymus method
[0049] Weigh 25g of PLGA (Mn=10000, LA:GA=75:25), add it into 100mL NMP solvent, mix well, and prepare a polymer solution, then add 3.5g of thymus method new raw material, heat and stir at constant temperature (temperature 30℃ , 100rpm) for 20 minutes to form a translucent drug-loaded sol system, which is sterilized by γ-ray radiation.
Embodiment 2
[0050] Example 2 Preparation of new in situ gel preparation of thymus method
[0051] Weigh 30g of PLGA (Mn=15000, LA:GA=50:50), add it to 100mL NMP solvent, mix well, and prepare a polymer solution, then add 3.5g of thymus method new raw material, heat and stir at constant temperature (temperature 30℃ , 120rpm) for 20 minutes to form a translucent drug-loaded sol system, which is sterilized by γ-ray radiation.
Embodiment 3
[0052] Example 3 Preparation of new in situ gel preparation of thymus method
[0053] Weigh 25g PLGA (Mn=20000, LA:GA=50:50) and 4g poloxamer 407, dissolve in 110mL NMP, mix well, and prepare a polymer solution, then add 3g thymofasin, heat and stir at constant temperature (Temperature 30°C, 100rpm) for 25 minutes to form a translucent drug-loaded sol system, which is sterilized by γ-ray radiation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com