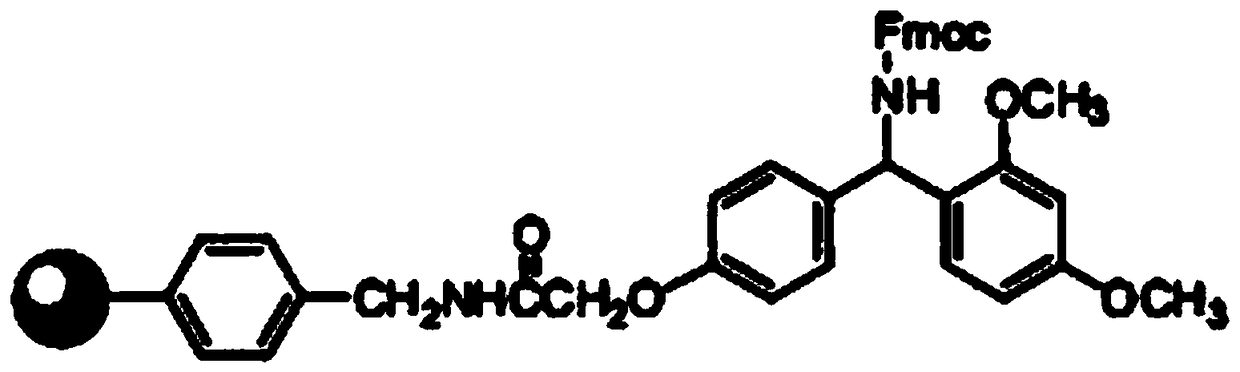
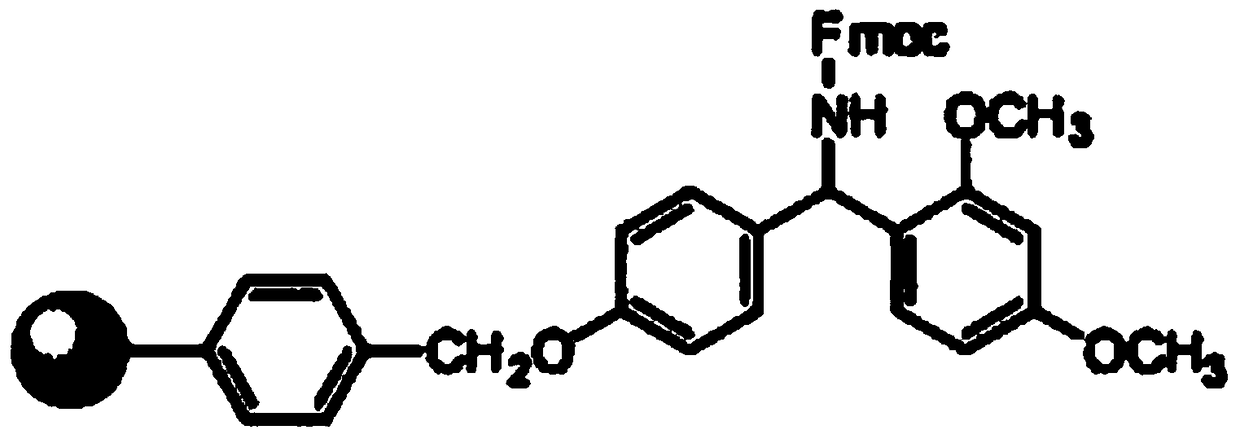
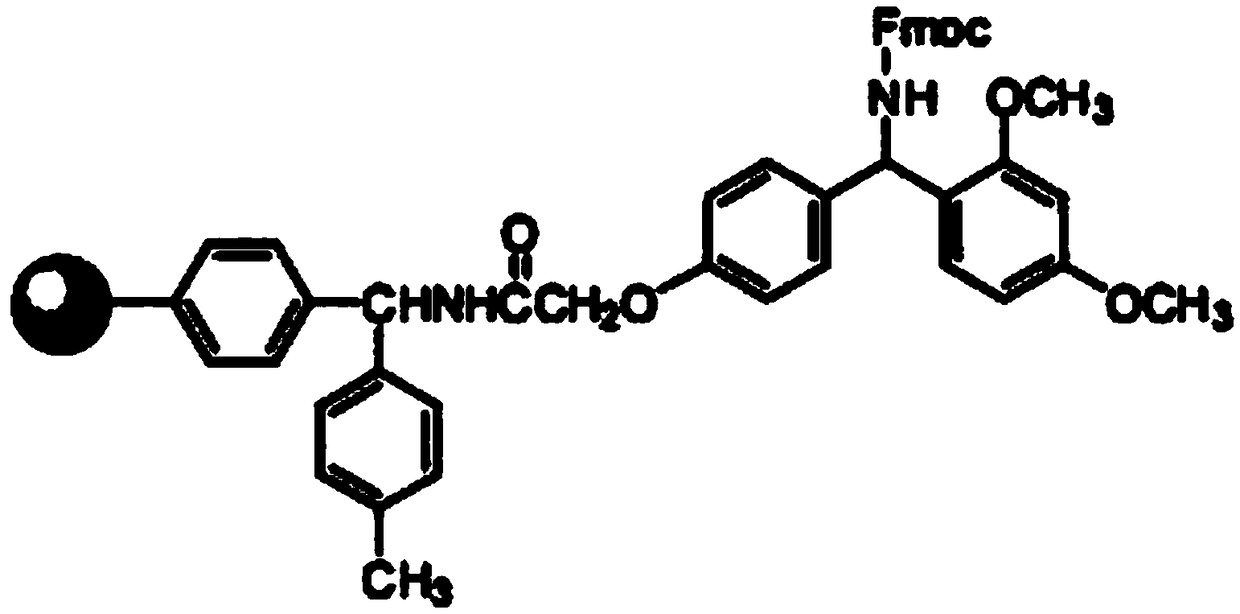
Synthesis method of thymalfasin
A new technology of thymus method and synthetic method, which is applied in the preparation method of peptides, chemical instruments and methods, animal/human proteins, etc., can solve the problem of reducing the yield of subsequent amino acid coupling, increasing the difficulty of separation and purification, and reducing product yield And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Synthesis of Peptide Fragment 1
[0088] Take by weighing the 2-CTC resin 0.5Kg that substitution degree is 0.6mol / g, join in the solid-phase synthesizer reactor, wash 2 times with DMF, drain after 30 minutes with DMF swelling resin, get 0.6mol Fmoc-Glu ( OtBu)-OH was dissolved in DMF, added to the above-mentioned reaction column equipped with resin, then added 1.2mol DIPEA, dried after 2 hours of reaction, added DMF solution containing 1.2mol of anhydrous methanol, stirred and reacted for 1 hour, washed with DMF 6 times to obtain Fmoc-Glu(OtBu)-2-CTC resin.
[0089] Use 10 mL of 20% piperidine / DMF solution per gram of resin to remove the Fmoc protecting group in the Fmoc-Glu(OtBu)-2-CTC resin for 20 minutes, then wash 6 times with DMF to obtain H-Glu(OtBu)-2-CTC resin.
[0090] Take 0.9 mol Fmoc-Lys(Boc)-OH and 0.9 mol HOBt, dissolve them in DMF, add 0.9 mol DIC under stirring, continue to stir for 1 hour, add to the reactor of solid-phase synthesizer, and react at r...
Embodiment 2
[0096] Synthesis of Peptide Fragment 1
[0097] Take by weighing the 2-CTC resin 0.5Kg that substitution degree is 0.6mol / g, join in the solid-phase synthesizer reactor, wash 2 times with DMF, drain after 30 minutes with DMF swelling resin, get 0.6mol Fmoc-Glu ( OtBu)-OH was dissolved in DMF, added to the above-mentioned reaction column equipped with resin, then added 1.2mol DIPEA, dried after 2 hours of reaction, added DMF solution containing 1.2mol of anhydrous methanol, stirred and reacted for 1 hour, washed with DMF 6 times to obtain Fmoc-Glu(OtBu)-2-CTC resin.
[0098] Use 10 mL of 20% piperidine / DMF solution per gram of resin to remove the Fmoc protecting group in the Fmoc-Glu(OtBu)-2-CTC resin for 20 minutes, then wash 6 times with DMF to obtain H-Glu(OtBu)-2-CTC resin.
[0099] Take 0.9 mol Fmoc-Lys(Boc)-OH and 0.9 mol HOBt, dissolve in DMF, add 0.86 mol HBTU under stirring, continue stirring for 1 hour, then add 1.3 mol DIPEA, mix well and add to the solid-phase syn...
Embodiment 3
[0105] Synthesis of Peptide Fragment 2
[0106] Weigh 0.2Kg of Rink MBHA resin with a degree of substitution of 0.5mol / g, add it to the reactor of a solid-phase synthesizer, wash it twice with DMF, swell the resin with DMF for 30 minutes, and drain it, and use 10mL of 20% piperidine per gram of resin / DMF solution to remove the Fmoc protecting group in the resin for 20 minutes, and then wash 6 times with DMF to obtain the deprotected Rink MBHA resin.
[0107] Take 0.3mol Asp(α-OtBu)-OH and 0.3mol HOBt, dissolve with DMF, add 0.3mol DIC under stirring, continue to stir and react for 1 hour, add it to the reactor of solid-phase synthesizer, and react at room temperature for 2h (reaction end point is indene The triketone method is used for detection, if the resin is colorless and transparent, the reaction is complete, and the resin develops color, indicating that the reaction is incomplete, and the coupling reaction time needs to be prolonged) to obtain Fmoc-Asp (α-OtBu)-Rink MBH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com