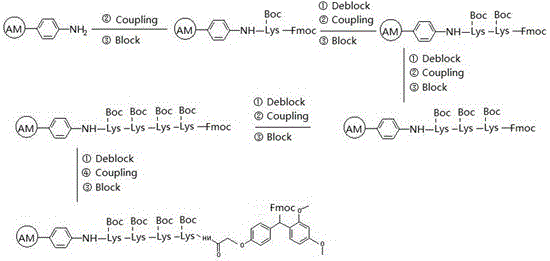
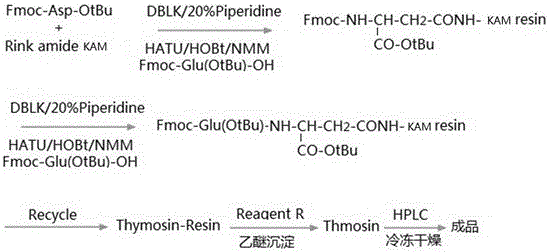
Synthesis process of thymalfasin
A thymic method, a synthesis process technology, is applied in the preparation methods of peptides, chemical instruments and methods, animal/human proteins, etc., and can solve the problems of decreased yield, high synthesis cost, low purity and yield, etc. The effect of eliminating beta sheet and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 Fmoc-Lys(Boc)-Lys(Boc)-Lys(Boc)-Lys(Boc)-AM resin synthesis
[0041] Weigh 10 g of AM resin with a substitution value of 1.1 mmol / g, puff it with DMF for 30 minutes, drain it, weigh 44 mmol Fmoc-Lys(Boc)-OH, 44 mmol HCTU and 44 mmol HOBt, measure 88 mmol DIEA, and use 60 Dissolve amino acid, HCTU and HOBt in milliliter DMF, add DIEA dropwise, mix well and cool in an ice bath, activate for 15 minutes, add puffed resin, react for 12 hours, stop the reaction after ninhydrin test is negative; prepare 60 milliliters of blocking solution ( 20 mmol acetic anhydride and 200 mmol triethylamine were dissolved in DMF, and the volume was adjusted to 60 ml), added to the resin, and reacted at room temperature for 1 hour. The blocked resin was deprotected with 50% piperidine DMF solution for 15 minutes, washed 8 times with DMF, and then the second coupling of Fmoc-Lys(Boc)-OH was performed, and the coupling was repeated to complete 4 Fmoc-Lys(Boc)- The substitution valu...
Embodiment 2
[0042] Example 2 Synthesis of Rink amide-Lys(Boc)-Lys(Boc)-Lys(Boc)-Lys(Boc)-AM resin
[0043] The resin prepared in Example 1 was deprotected by adding 20% piperidine DMF solution. The deprotection time was 15 minutes. After the deprotection was completed, the DMF was washed 8 times until it was completely clean; 4 mmol Rink amide Linker, 4 mmol HCTU, and 8 mmol DIEA were weighed , Dissolve Rink amide Linker and HCTU with 10 ml of DMF, add DIEA dropwise, mix well and cool in an ice bath, add deprotected resin after 15 minutes of activation, increase DMF until the resin is completely covered, react for 3 hours, and seal with 10 ml of blocking solution After 30 minutes, freeze-dry after washing, and determine the substitution value, which is 0.12 mmol / g.
Embodiment 3
[0044] Example 3 Synthesis of Fmoc-Asp(Rink amide-Lys(Boc)-Lys(Boc)-Lys(Boc)-Lys(Boc)-AM)-OtBu
[0045] Weigh 8.3 g of the Rink amide-Lys(Boc)-Lys(Boc)-Lys(Boc)-Lys(Boc)-AM resin prepared in Example 2, add 20% piperidine DMF solution for deprotection, and the deprotection time is 15 minutes , after the deprotection is completed, wash with DMF 8 times until completely clean; weigh 4.115 g of Fmoc-Asp-OtBu, 4.137 g of HCTU, and 1.35 g of HOBt, add 15 ml of DMF to dissolve, add dropwise 3.3 ml of DIEA, activate in ice bath for 15 minutes, add Coupling in the prepared resin for 2 hours, the ninhydrin reagent detects that the resin is colorless and transparent, indicating that the reaction is complete, and the resin is washed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com