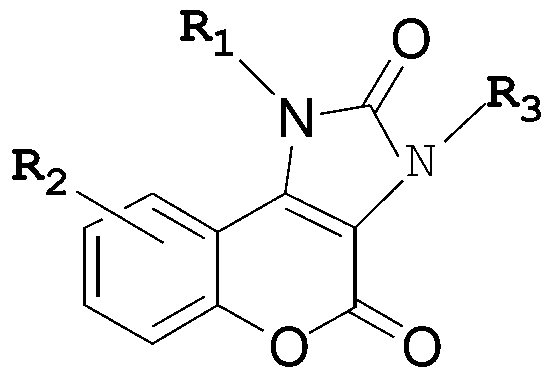
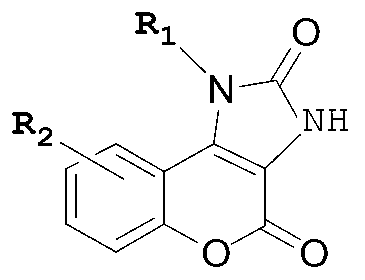
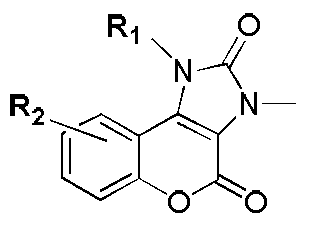
Five-membered urea ring-coumarin derivative or pharmaceutical salt and application thereof
A technology of medicinal salt and halogen, which is applied in the field of chemical medicine and can solve problems such as large side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Compound Ia: 2-methyl-2-[4-[2-oxo-8-bromo-2,3-dihydroimidazo[4,5-c]coumarin-1-yl]benzene Base] the preparation of propionitrile
[0062]
[0063] Intermediates involved:
[0064] The synthetic method of this intermediate is as follows:
[0065]
[0066] 1, the preparation of 4-bromophenyl acetate:
[0067] Add 4-bromophenol (97.87g, 0.566mol) into 80mL of acetic anhydride (86.6g, 0.849mol), add 91mL of pyridine (89.43g, 1.132mol), heat the reaction solution to 100°C, stir for 4 hours, and use Adjust dilute hydrochloric acid to slightly acidic, and then extract 3 times with ethyl acetate, adjust the organic layer to weak alkaline with saturated sodium bicarbonate solution, wash 3 times with water, anhydrous MgSO 4 After drying and concentrating, 122 g of the product was obtained, and the yield was 100%.
[0068] The preparation of 4-bromophenylacetate is also commercially available.
[0069] 2. Preparation of 2-hydroxyl-5-bromoacetophenone:
[007...
Embodiment 2
[0099] Example 2 Compound Ib: 2-methyl-2-[4-[2-oxo-8-(quinolin-3-yl)-2,3-dihydroimidazo[4,5-c]coumarin Preparation of prime-1-yl]phenyl]propionitrile
[0100]
[0101] 2-Methyl-2-[4-(2-oxo-8-bromo-2,3-dihydroimidazo[4,5-c]coumarin-1-yl)phenyl]propionitrile ( 214 mg, 0.51 mmol) and quinoline-3-boronic acid (100 mg, 0.56 mmol) were mixed in 40 mL of dioxane: water = 3:1 solution, and then potassium carbonate (210 mg, 1.52 mmol) and PdCl were added 2 (dppf) (20mg, 0.02mmol), under nitrogen protection, react at 65°C. After 4 hours, the reaction solution was filtered, the filtrate was concentrated, and the product was separated by column chromatography (115 mg, yield 48%).
[0102] 1 H-NMR (DMSO-d 6 , 400MHz): 2.65 (s, 6H), 6.82 (d, J=1.6Hz, 1H), 7.65 (t, J=7.4Hz, 1H), 7.71 (d, J=8.4Hz, 1H), 7.78 (t , J=7.8Hz, 1H), 7.86 (d, J=8.4Hz, 2H), 7.98 (m, 5H), 8.28 (s, 1H), 8.71 (d, J=2.0Hz, 1H) ppm.
Embodiment 3
[0103] Example 3 Compound IIa: Preparation of 2-[4-(2-oxo-8-bromo-2,3-dihydroimidazo[4,5-c]coumarin-1-yl)phenyl]acetonitrile
[0104]
[0105] 1. Preparation of 2-[4-N-(3-nitro-6-bromocoumarin-4-yl)phenyl]acetonitrile
[0106] Method with reference to embodiment 1.
[0107] 1 H-NMR (CDCl 3 , 400MHz): 4.03 (s, 2H), 5.30 (s, 1H), 7.30 (d, J=8.8Hz, 1H), 7.71 (dd, J=2.4Hz, 9.6Hz, 1H), 7.88 (dd, J =2.0Hz, 9.2Hz, 1H), 8.05 (s, 1H), 8.30 (d, J=8.8Hz, 1H), 8.91 (d, J=2.4Hz, 1H), 9.23 (s, 1H) ppm.
[0108] 2. Preparation of 2-[4-N-(3-amino-6-bromocoumarin-4-yl)phenyl]acetonitrile
[0109] Method with reference to embodiment 1.
[0110] 1 H-NMR (CDCl 3 , 400MHz): 3.19 (s, 2H), 4.23 (br, s, 2H), 5.44 (s, 1H), 6.71 (d, J=8.4Hz, 2H), 7.25 (m, 3H), 7.43 (dd, J=2.2Hz, 8.6Hz, 1H), 7.53 (d, J=2.4Hz, 1H) ppm.
[0111] 3. Preparation of 2-[4-(2-oxo-8-bromo-2,3-dihydroimidazo[4,5-c]coumarin-1-yl)phenyl]acetonitrile
[0112] Method with reference to embodiment 1.
[0113] 1 H-NMR (D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com