Method for detecting impurities in choline alfoscerate
A technology of choline alfoscerate and a detection method, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of affecting the quality of medicines and not being able to effectively detect impurities in choline alfoscerate, and achieve good repeatability and stability performance, improve quality monitoring standards, and effectively monitor the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0183] Mix choline alfoscerate, choline chloride, glycerol phosphatidylinositol, empty capsule shell and glycerin with water, and filter after mixing evenly to obtain choline alphosate mixed solution A. Contains choline alfosate 50mg, choline chloride 0.25mg, glycerol phosphatidylinositol 0.25mg, empty capsule shell 50mg, glycerin 3mg.
[0184] Take 20 μL of the choline alfoscerate mixed solution A, inject it into a Shimadzu liquid chromatograph, and use an Alltech 2000ES evaporative light scattering detector for detection.
[0185] Shimadzu liquid chromatograph adopts LC-10ATvp pump, CBM-20A controller, CLASS-VP chromatography workstation;
[0186] The drift tube temperature of the Alltech 2000ES evaporative light scattering detector is 80°C, the atomizing gas is nitrogen, the atomizing gas flow rate is 2.5L / min, and the carrier gas pressure is 0.15MPa;
[0187] The chromatographic conditions are: Shiseido CAPCELL PAK SCX chromatographic column, the particle size of the chro...
Embodiment 2
[0198] Take 500 mg of the choline alfoscerate sample, place it in a 10 ml volumetric flask, add water to dissolve and constant volume, and shake evenly to obtain the choline alfoscerate sample solution to be tested.
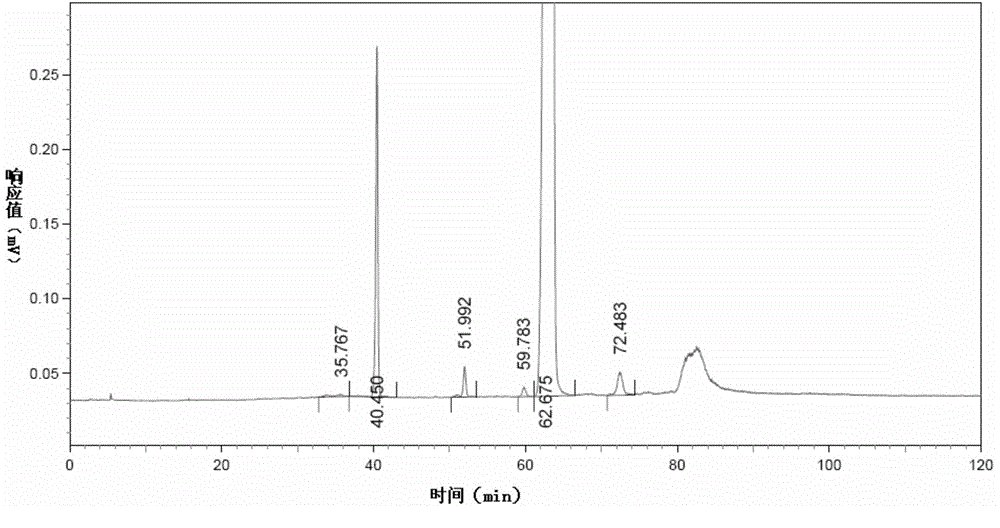
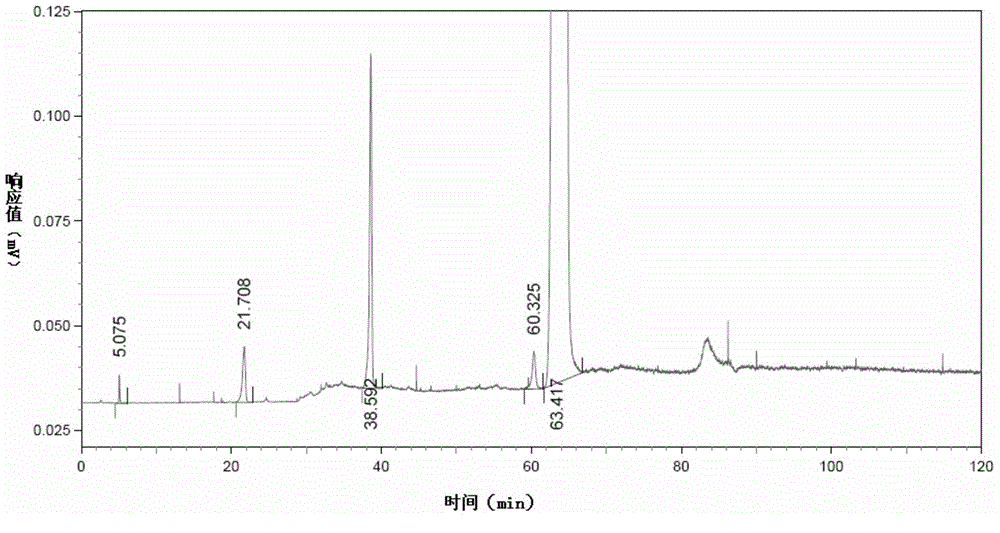
[0199] Measure 20 μ L of choline alfoscerate test sample solution, detect according to the liquid chromatography detection method in the embodiment of the present invention 1, obtain the first liquid phase chromatogram of test sample solution of choline alfoscerate, as figure 2 as shown, figure 2 It is the first liquid chromatogram of the choline alfoscerate test sample solution obtained in Example 2 of the present invention. Depend on figure 2 Can obtain the retention time of main impurity in the choline alfoscerate sample, the result is as shown in table 3, and table 3 is in the choline alfoscerate test sample solution that the embodiment of the present invention 2,3,5,7,9 and 11 obtain Retention time of major impurities.
Embodiment 3
[0201] Take 500mg of choline alfoscerate sample, place it in a 10ml volumetric flask, add 2ml of 1mol / L hydrochloric acid solution, place it in a water bath at 40°C for 1 hour, add 1mol / L sodium hydroxide solution to dissolve the solution in the volumetric flask Neutralize to neutral, then add water to dissolve and constant volume, shake evenly and filter, take the subsequent filtrate to obtain the sample solution of choline alfoscerate to be tested.
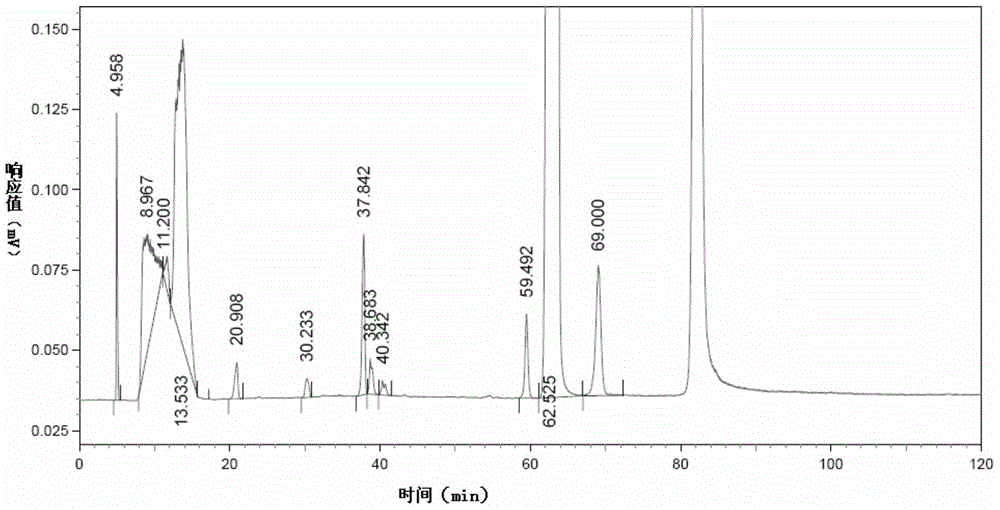
[0202] Measure 20 μ L of choline alfoscerate solution to be tested, and detect it according to the liquid chromatography detection method in Example 1 of the present invention. The difference is that the volume ratio of mobile phase B acetonitrile and ammonium formate solution in this example is 35 :65. Obtain the first liquid phase chromatogram of choline alfoscerate test sample solution, as image 3 as shown, image 3 It is the first liquid chromatogram of the choline alfoscerate test sample solution obtained in Example 3 of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com