Urapidil hydrochloride injection and preparation method thereof
A technology of urapidil hydrochloride and injection, which is applied in the field of medicine, can solve problems such as greater influence of temperature, influence on product quality, and influence on product efficacy, so as to avoid pollution, optimize pH value stability, and improve sterility assurance level Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
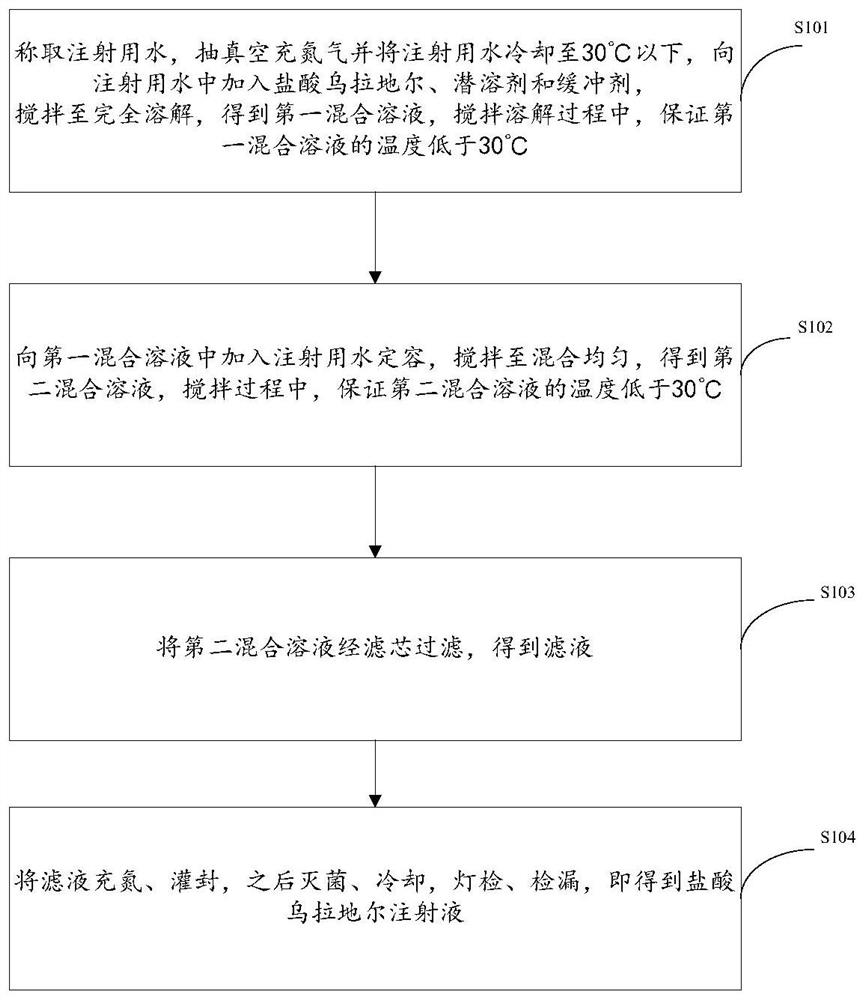
[0028] figure 1 It is a schematic flow sheet of the preparation method of urapidil hydrochloride injection provided by the embodiment of the application, refer to figure 1 , the preparation method provided by the application comprises the following steps:
[0029] Step S101, weighing the water for injection, vacuuming and filling with nitrogen and cooling the water for injection to below 30°C, adding urapidil hydrochloride, co-solvent and buffer to the water for injection, stirring until completely dissolved to obtain the first mixed solution, stirring During the dissolution process, cooling water is passed into the jacket during the liquid preparation process to ensure that the temperature of the first mixed solution is lower than 30°C; the latent solvent is one of ethanol, 1,2-propylene glycol, glycerin, polyethylene glycol or Several kinds; the buffer is a mixture of potassium dihydrogen phosphate / potassium chloride / sodium chloride, a mixture of disodium hydrogen phosphate...
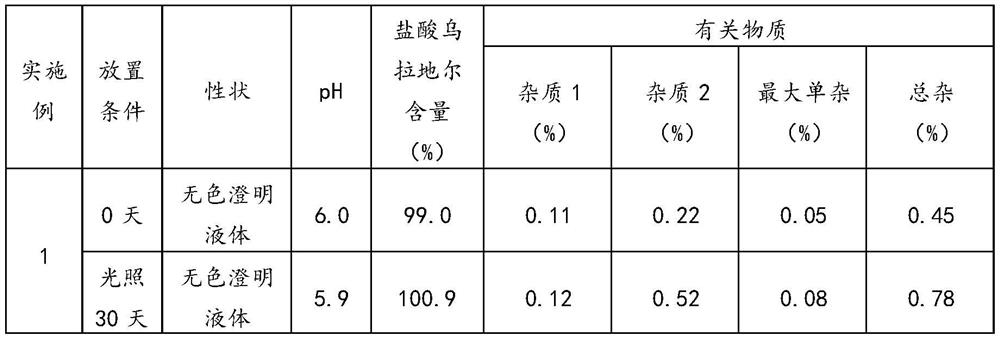
Embodiment 1
[0036] Embodiment 1 of the present application provides a preparation method of urapidil hydrochloride injection, comprising the following steps:
[0037] (1), take by weighing 50%-80% of the water for injection of the full prescription, vacuumize and fill with nitrogen, and cool the water for injection so that the temperature is lower than 10°C;
[0038] (2), add 5.47g urapidil hydrochloride in water for injection, stir until dissolving completely, obtain urapidil hydrochloride solution, pass into cooling water in the jacket of liquid holding container in stirring process and guarantee that the temperature of medicinal solution is lower than 10°C;
[0039] (3), add 0.28g disodium hydrogen phosphate, 1.8g sodium dihydrogen phosphate to urapidil hydrochloride solution, stir until completely dissolved, obtain the first mixed solution, pass into the jacket of the liquid container during stirring Cooling water ensures that the temperature of the liquid medicine is lower than 10°C...
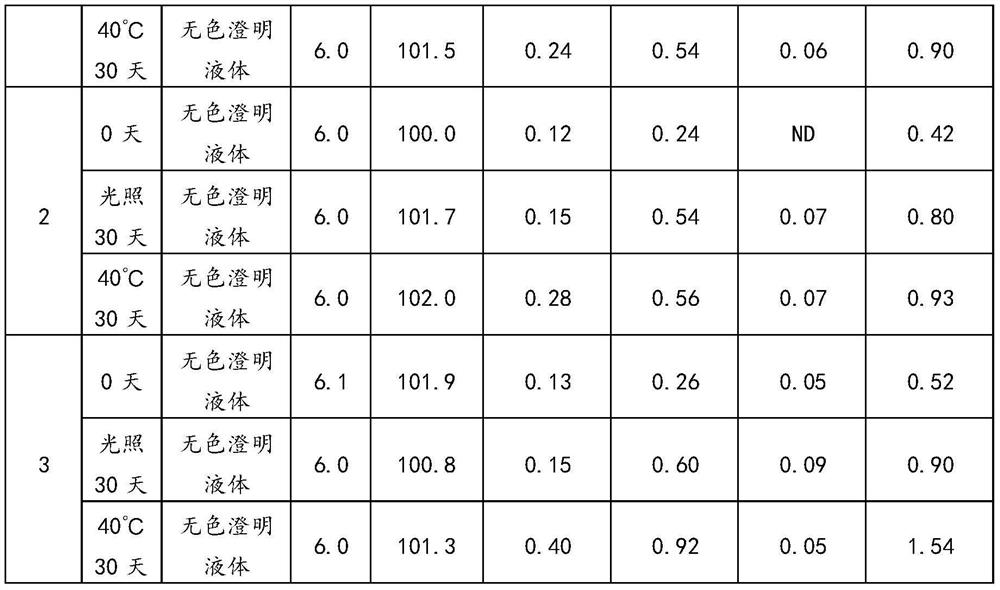
Embodiment 2
[0044]Embodiment 2 of the present application provides a kind of preparation method of urapidil hydrochloride injection, comprises the following steps:
[0045] (1), take by weighing 50%-80% of the water for injection of the full prescription, vacuumize and fill with nitrogen, and cool the water for injection so that the temperature is lower than 30°C;
[0046] (2), add 5.47g urapidil hydrochloride in water for injection, stir until dissolving completely, obtain urapidil hydrochloride solution, pass into cooling water in the jacket of liquid holding container in stirring process and guarantee that the temperature of medicinal solution is lower than 30°C;
[0047] (3), add 100g 1,2-propanediol, 0.28g disodium hydrogen phosphate, 1.8g sodium dihydrogen phosphate to the urapidil hydrochloride solution, stir until completely dissolved to obtain the first mixed solution, in the stirring process Cooling water is passed into the jacket of the container to ensure that the temperature...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com