Urapidil hydrochloride injection and preparation method thereof
A technology of urapidil hydrochloride and injection, which can be applied to pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc. It can improve the stability, reduce the probability of deterioration, and promote the effect of large-scale industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
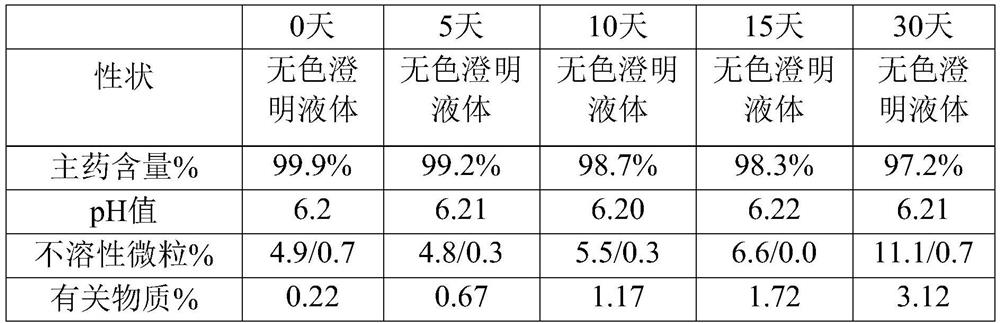
[0033] The embodiment of the present invention provides a kind of urapidil hydrochloride injection, and every 1000mL of urapidil hydrochloride injection contains the following raw materials: 5 g of urapidil hydrochloride, 100 g of polyethylene glycol, 0.3 g of citric acid and 2.6 g of sodium citrate g, the pH value of the urapidil hydrochloride injection is 6.2.
[0034] The preparation method of above-mentioned urapidil hydrochloride injection comprises the following steps:
[0035] Step 1: weighing each component according to the above-mentioned raw material ratio;
[0036] Step 2: Cool down the water for injection of 60% of the total volume of the preparation to 50° C., under nitrogen protection, add urapidil hydrochloride, polyethylene glycol, citric acid and sodium citrate, dissolve evenly and add at a temperature of The remaining water for injection at 50°C was adjusted to a pH value of 6.2, filtered through a polyethersulfone filter element with a pore size of 0.45 μm ...
Embodiment 2
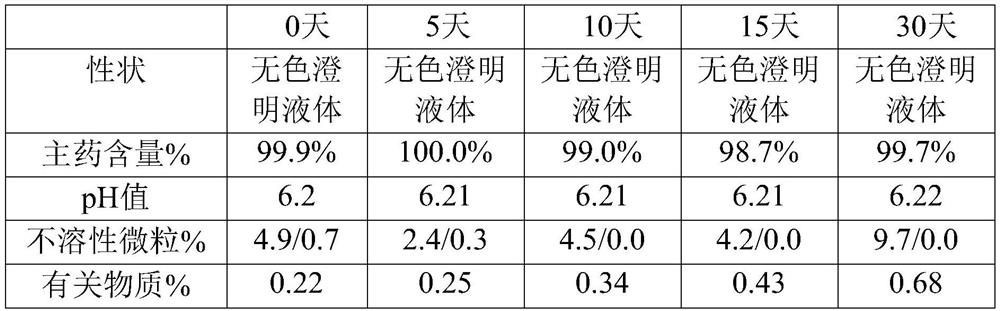
[0039] The embodiment of the present invention provides a kind of urapidil hydrochloride injection, and every 1000mL of urapidil hydrochloride injection contains the following raw materials: 5 g of urapidil hydrochloride, 100 g of polyethylene glycol, 0.3 g of citric acid and 3.3 g of sodium citrate g, the pH value of the urapidil hydrochloride injection is 6.3.
[0040] The preparation method of above-mentioned urapidil hydrochloride injection comprises the following steps:
[0041] Step 1: weighing each component according to the above-mentioned raw material ratio;
[0042] Step 2: The water for injection of 80% of the total volume of the preparation is cooled to 30°C, under the protection of nitrogen, add urapidil hydrochloride, polyethylene glycol, citric acid and sodium citrate, and add the temperature after dissolving evenly. For the remaining water for injection at 45°C, adjust the pH value to 6.3, filter through a polyethersulfone filter element with a pore size of 0....
Embodiment 3
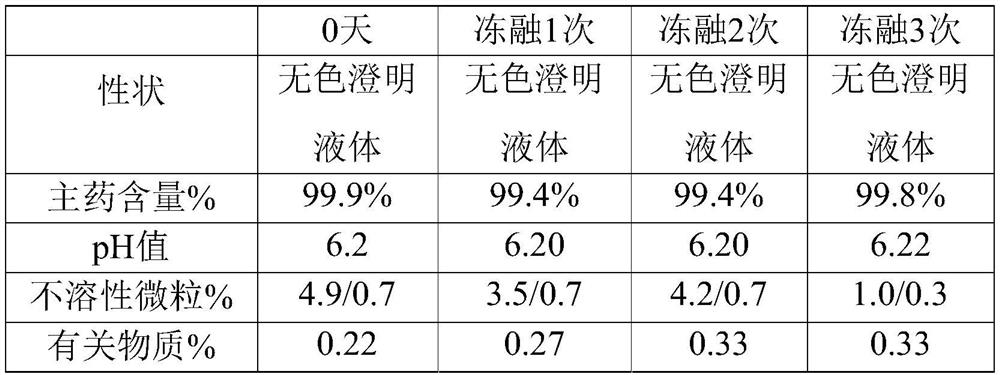
[0045] The embodiment of the present invention provides a kind of urapidil hydrochloride injection, and every 1000mL of urapidil hydrochloride injection contains the following raw materials: 5 g of urapidil hydrochloride, 100 g of polyethylene glycol, 0.3 g of citric acid and 2.3 g of sodium citrate g, the pH value of the urapidil hydrochloride injection is 6.1.
[0046] The preparation method of above-mentioned urapidil hydrochloride injection comprises the following steps:
[0047] Step 1: weighing each component according to the above-mentioned raw material ratio;
[0048] Step 2: Cool down the water for injection of 70% of the prepared total volume to 35° C., under nitrogen protection, add urapidil hydrochloride, polyethylene glycol, citric acid and sodium citrate, dissolve evenly and add at a temperature of For the remaining water for injection at 40°C, adjust the pH value to 6.1, filter through a polyethersulfone filter element with a pore size of 0.45 μm and a polyethe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com