Urapidil sustained release preparation and preparation method thereof
A technology of urapidil and sustained-release preparations, which is applied in the field of urapidil sustained-release preparations and its preparation, can solve the problems of inability to release drugs continuously, and achieve superior protective effects, no sudden release phenomenon, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] On the other hand, the present invention also provides a preparation method of urapidil sustained-release preparation, comprising the steps of:
[0060] Prepare drug-containing pellets, the step of preparing drug-containing pellets includes:
[0061] Step S10, weighing the adhesive according to the prescription amount and dissolving it in the solvent at the first temperature, and then adding an anti-adhesive agent to obtain an adhesive solution;
[0062] Step S20, weighing the urapidil and the diluent according to the prescription amount and passing through the first-mesh sieve to obtain the urapidil and the diluent meeting the particle size requirements;
[0063] Step S30, mixing the urapidil meeting the particle size requirement with the diluent, and mixing uniformly to obtain the first mixed powder;
[0064] Step S40, weighing the blank core of the first mass and placing it in a centrifugal coating granulator, and then adding the binder solution and the first mixed ...
Embodiment 1
[0091] In order to study the effect of the isolation layer on the dissolution of sustained-release capsules under high temperature conditions, two kinds of pellets were prepared: drug-containing pellets without isolation layer and drug-containing pellets with isolation layer B, and then poured into capsules to obtain sustained-release capsules A and sustained-release capsules. Capsule B was placed in a closed container at a high temperature of 60°C at the same time, and the dissolution results were investigated for 10 days.
[0092] One, the preparation of sustained-release capsule A
[0093] 1. Preparation of drug-containing pellets:
[0094] Medicated pellets contain the following ingredients:
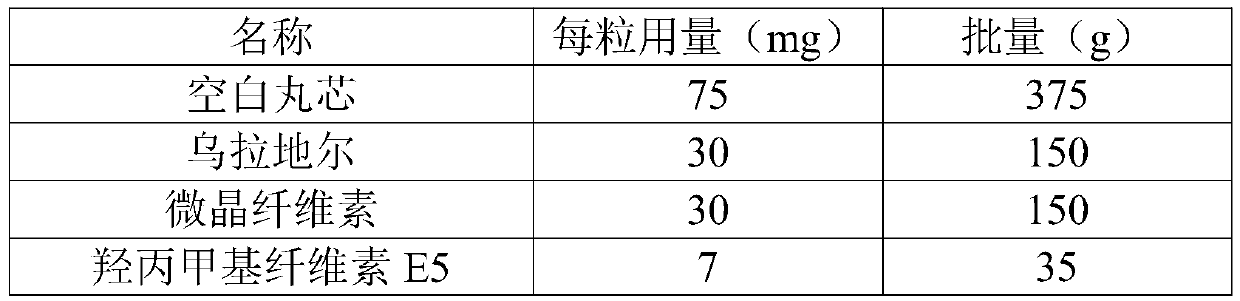
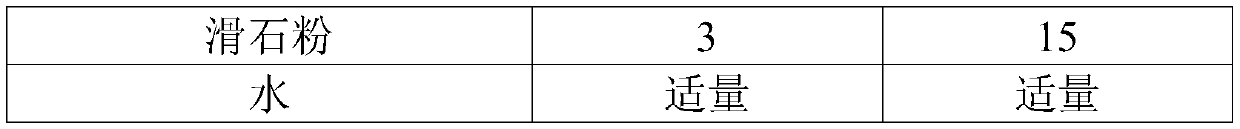
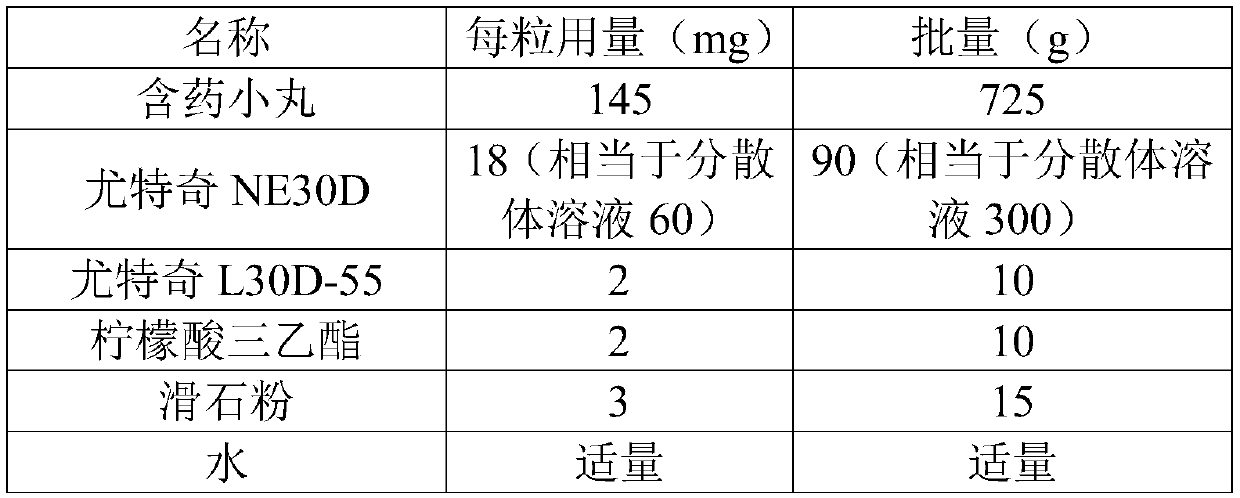
[0095]
[0096]
[0097] Described preparation process comprises the steps:
[0098] Step S1: Add hypromellose to a container filled with purified water at 80°C to 90°C, stir to disperse, let cool, then stir, then add talcum powder to obtain a 3% hypromellose solution;
[00...
Embodiment 2
[0144] 1. Preparation of drug-containing pellets:
[0145] Medicated pellets contain the following ingredients:
[0146] name Dosage per capsule (mg) Batch (g) blank core 75 375 Uradil 30 150 microcrystalline cellulose 30 150 Povidone K30 7 35 talcum powder 3 15 water Appropriate amount Appropriate amount
[0147] Described preparation process comprises the steps:
[0148] Step S1: Weigh Povidone K30 according to the prescription amount and add it into a container filled with purified water at 80°C to 90°C, stir to disperse, let cool, then stir, and then add talcum powder to obtain a 3% Povidone K30 solution;
[0149] Step S2: Weigh urapidil and microcrystalline cellulose according to the prescription and pass through a 100-mesh sieve, and mix the two substances evenly to obtain the first mixed powder;
[0150] Step S3: Weigh 500g of the blank pellet core and place it in the centrifugal coating granulator, set the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com