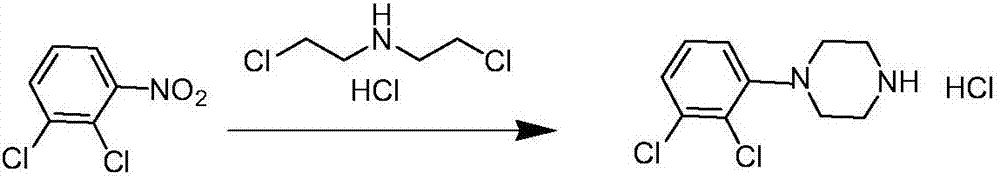
Preparation method of aripiprazole intermediate 1-(2,3-dichlorophenyl)piperazine hydrochloride
A technology of aripiprazole and dichlorophenyl is applied in the field of preparation of aripiprazole intermediate 1-piperazine hydrochloride, and can solve the problems of unsuitability for large-scale production, long reaction time, low yield and the like , to achieve the effect of low cost, simple operation and less waste liquid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In a 500ml reaction bottle, add 10g 2,3 dichloronitrobenzene, 9.3g bis(2-chloroethyl)amine hydrochloride, add 100ml diethylene glycol ether, 8.7g iron powder, 14.0g ammonium chloride , 125ml purified water, 0.90g potassium iodide, 1.68g tetrabutylammonium bromide, heat up to 130°C, keep warm for 20h, stop heating, cool down to room temperature and filter with suction, add 100ml of acetone to the mother liquor and stir for 1.5h, crystallize, and filter with suction to obtain The crude product was dried to obtain 9.47g of solid, purity (HPLC): 99.0%, yield 68.0%; ESI (m / z): 266.01.
Embodiment 2
[0021] In a 500ml reaction flask, add 10g 2,3 dichloronitrobenzene, 9.8g bis(2-chloroethyl)amine hydrochloride, add 100ml diethylene glycol ether, 10.5g iron powder, 14.0g ammonium chloride , 125ml of purified water, 1.0g of potassium iodide, 1.92g of tetrabutylammonium bromide, heat up to 130°C, keep warm for 20h, stop heating, cool down to room temperature and filter with suction, add 100ml of acetone to the mother liquor and stir for 1.5h, crystallize, and filter with suction to obtain The crude product was dried to obtain 9.52 g of solid, purity (HPLC): 99.2%, yield 68.3%; ESI (m / z): 266.01.
Embodiment 3
[0023] In a 500ml reaction bottle, add 10g 2,3 dichloronitrobenzene, 10.2g bis(2-chloroethyl)amine hydrochloride, add 100ml diethylene glycol ether, 12.0g iron powder, 14.0g ammonium chloride , 125ml purified water, 1.10g potassium iodide, 2.14g tetrabutylammonium bromide, heat up to 130°C, keep warm for 20h, stop heating, cool down to room temperature and filter with suction, add 100ml of acetone to the mother liquor and stir for 1.5h, crystallize, and filter with suction to obtain The crude product was dried to obtain a solid 9.67g, purity (HPLC): 99.4%, yield 69.4%; ESI (m / z): 266.01.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com