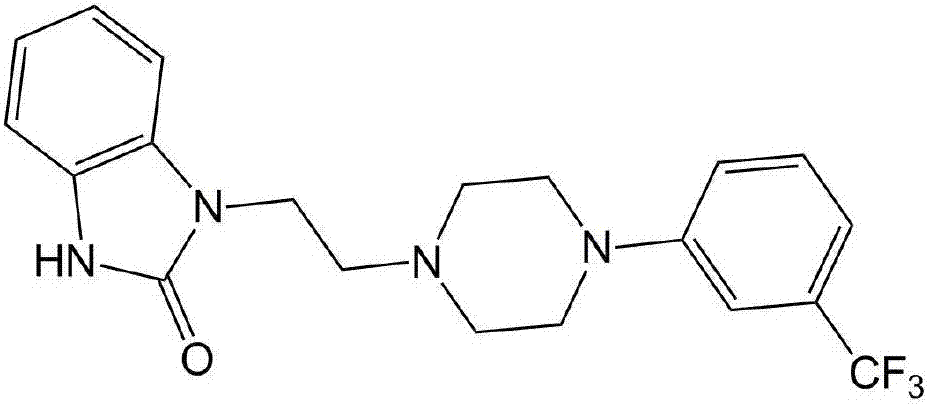
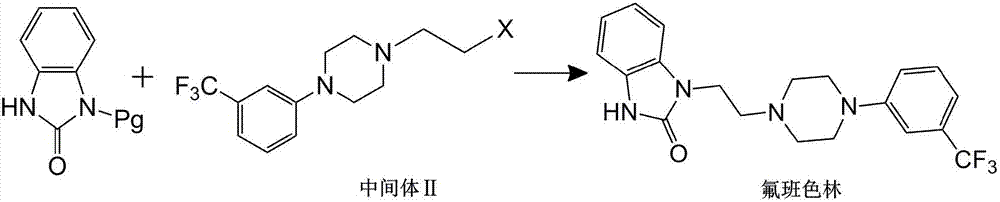
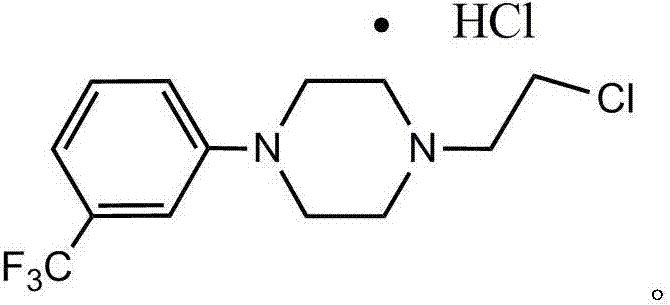
Preparation method of flibaserin intermediate
A flibanserin and intermediate technology, which is applied in the field of preparation of flibanserin intermediates, can solve the problems of many side reactions, low reaction selectivity, high irritation and the like, and achieves improved reaction selectivity and product yield High, easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of 2-(4-(3-trifluoromethylphenyl)piperazine-1-ethanol
[0027] (1) Add 1-(3-trifluoromethylphenyl)piperazine hydrochloride (35.0g, 0.131mol), NaOH (13.1g, 0.328mol) and 280ml of ethanol into a 250ml reaction flask, and stir;
[0028] (2) Add 2-bromoethanol (32.8g, 0.26mol), heat to reflux, and monitor the end point of the reaction with thin-layer chromatography;
[0029] (3) Filter the reactant, wash the filter residue with 100ml ethanol, combine the eluate and the filtrate, concentrate under reduced pressure, add water to the concentrated gain, and add NaOH to adjust the pH to be greater than 11, and stir;
[0030] (4) Extract with dichloromethane (150ml*2), extract twice, combine the organic layers, wash with saline (200ml*2), and then dry with anhydrous sodium sulfate;
[0031] (5) Filtration, concentration under reduced pressure to obtain 34.6 g of yellow oily substance 2-(4-(3-trifluoromethylphenyl)piperazine-1-ethanol, the yield reached 96% after dete...
Embodiment 2~16
[0033] The amount of each raw material in Examples 2 to 16, the process parameters of the preparation process and the 2-(4-(3-trifluoromethylphenyl)piperazine-1-ethanol output and yield are as shown in Table 1:
[0034] Table 1
[0035]
Embodiment 17
[0037] Preparation of Intermediate II
[0038] (1) Add 2-(4-(3-trifluoromethylphenyl)piperazine-1-ethanol (33.0g, 0.12mol), DCM (330ml) into a 500ml reaction flask, stir, and cool in an ice-water bath. This patent The DCM used in is dichloromethane;
[0039] (2) DCM solution (30ml) of thionyl chloride (57.0g, 0.479mol) was added dropwise, and the temperature was raised to reflux for 2h after the addition;
[0040] (3) Concentrate under reduced pressure after the reaction is completed, add ethanol to the concentrated product for recrystallization, and obtain 38.0 g of white solid powder, and the yield of intermediate II reaches 96% after detection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com