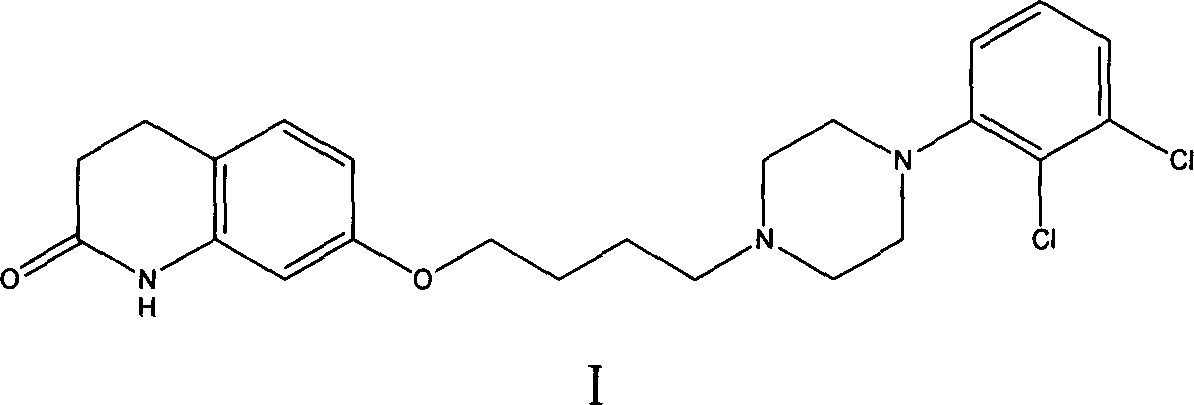
New synthesis method of aripiprazole
A technology of aripiprazole and piperazine, which is applied in the field of preparation of antipsychotic drug aripiprazole, can solve the problem of lower yield and purity of intermediate 4-bromobutoxyquinolinone, expensive reagents, and purification process cumbersome and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of 4-chlorobutyl p-toluenesulfonate
[0029] Add 4 g of anhydrous zinc chloride, 76 g of p-toluenesulfonyl chloride, and 20 mL of toluene into a 250 ml three-necked flask, stir and heat to 80°C, add 37.5 g of tetrahydrofuran dropwise, keep warm for 2 hours after the dripping, and pour the reaction solution into ice water. Add toluene to make the organic reverse phase to the upper layer, separate the water layer, wash the organic phase with sodium bicarbonate solution to neutrality, then wash with saturated brine, dry over anhydrous magnesium sulfate, recover toluene by distillation under reduced pressure, and decompress the oil pump Distilled to obtain 77 grams of 4-chlorobutyl p-toluenesulfonate, yield 73%, purity 98%
Embodiment 2
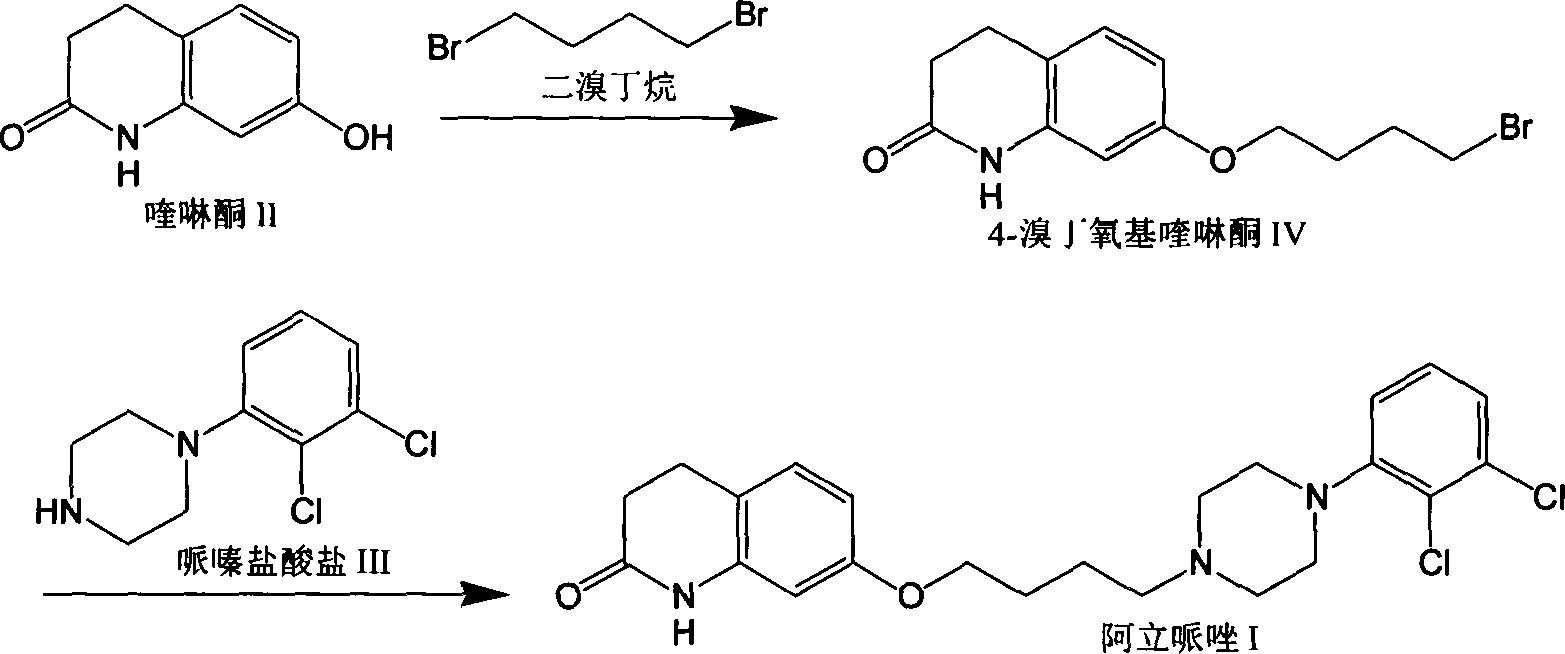
[0031] 4-Bromobutoxyquinolinone
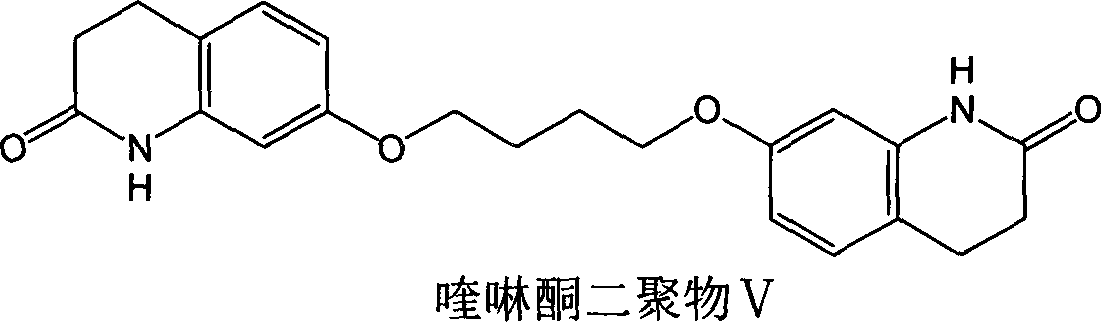
[0032] Add dehydrated ethanol 30ml, potassium carbonate 2.5 grams (18mmol), 7-hydroxyquinolinone 2.45 grams (15mmol), 1,4-dibromobutane 9.7 grams (45mmol) respectively in the there-necked flask, heating reaction for a period of time, TLC Or HPLC monitoring to the end of the reaction. After the reaction was completed, after concentrating under reduced pressure to recover most of the ethanol, 30ml of water was added to the reaction flask, and after stirring for a period of time, a solid was precipitated, filtered by suction, and washed to obtain 4.08 grams of crude product of 4-bromobutoxyquinolinone, dimer Content 17%, HPLC purity 78.6%.
Embodiment 3
[0034] 4-Chlorobutoxyquinolinone
[0035] 1. Add 30ml of absolute ethanol, 2.5 grams (18mmol) of potassium carbonate, 2.45 grams (15mmol) of 7-hydroxyquinolinone, and 11.8 grams (45mmol) of 4-chlorobutyl p-toluenesulfonate to the three-necked flask, and heat to react 2 Hours, TLC or HPLC monitoring to the end of the reaction. After the reaction was completed, after concentrating under reduced pressure to recover most of the ethanol, 30ml of water was added to the reaction flask, and after stirring for a period of time, a solid was precipitated, filtered by suction, and washed to obtain 3.21 grams of crude product of 4-chlorobutoxyquinolinone, dimer Content 0.7%, HPLC purity 97.3%.
[0036] 2. Add 30ml of absolute ethanol, 0.72 grams (18mmol) of sodium hydroxide, 2.45 grams (15mmol) of 7-hydroxyquinolinone, and 11.8 grams (45mmol) of 4-chlorobutyl p-toluenesulfonate to the three-necked flask, and heat the reaction After 1.5 hours, TLC or HPLC monitored to the end of the react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com