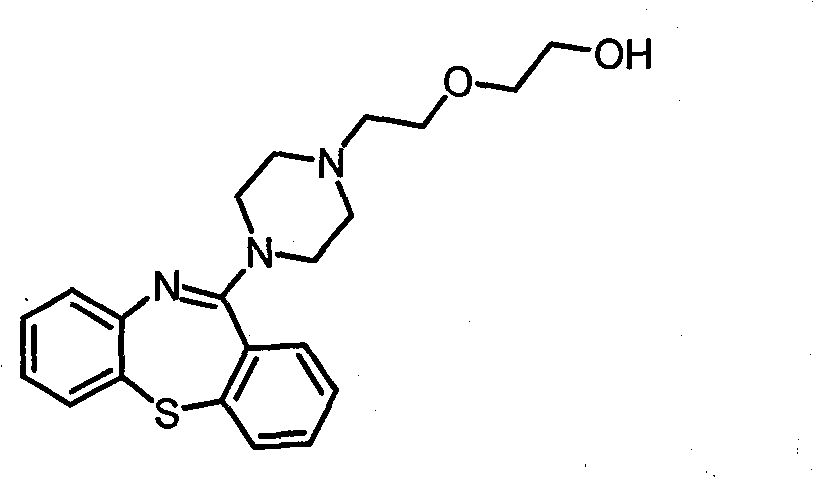
Novel preparation method of quetiapine
A kind of technology of quetiapine and piperazine, applied in directions such as carboxylate preparation, organic chemistry, etc., can solve the problems of time-consuming, difficult to adopt, long route and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
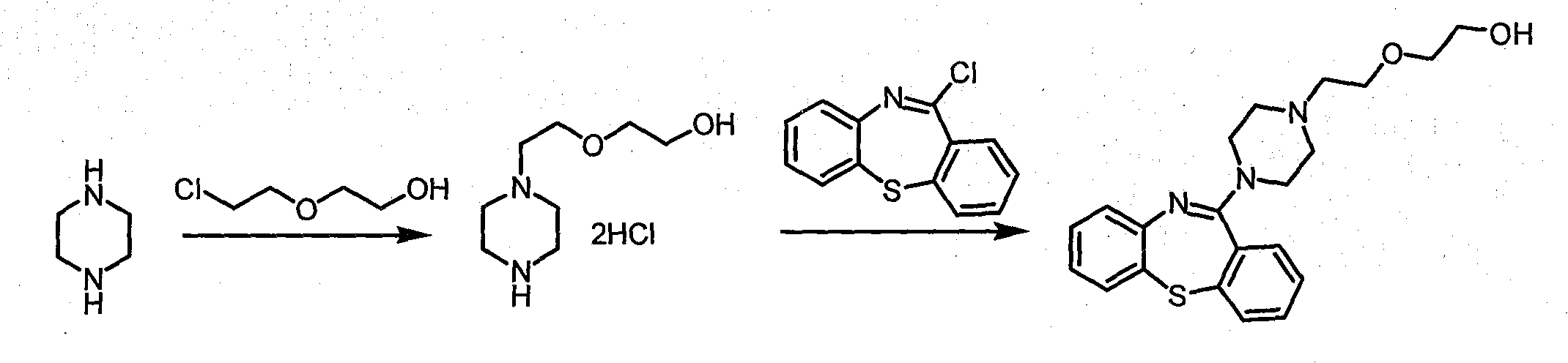
[0017] Heat 100 grams (1.2mol) of anhydrous piperazine and 240 grams (1.5mol) of piperazine dihydrochloride to 120 degrees Celsius, add dropwise 150 grams (1.2mol) of 2-(2-chloroethoxy)ethanol, add Continue heating and stirring after completion, raise the temperature to 136-140 degrees Celsius, and keep it for 1 hour, and stop heating after TLC shows that the reaction is complete. When the temperature drops to 80 degrees Celsius, add 500 milliliters of 95% ethanol, cool overnight in the refrigerator, filter and recover piperazine dihydrochloride the next day, wash the filter cake fully with a small amount of ethanol, combine the filtrates, and add 30% sodium hydroxide solution 200 grams of alkalization, filtered off insoluble inorganic salts; concentrated to remove the solvent, extracted the residue with ethyl acetate, passed through dry hydrogen chloride gas, filtered and dried to obtain a white solid 1-[2-(2-hydroxyethoxy Base) ethyl] piperazine hydrochloride; the solid cont...
example 2
[0019] Heat 100 grams (1.2mol) of anhydrous piperazine and 240 grams (1.5mol) of piperazine dihydrochloride to 120 degrees Celsius, add dropwise 150 grams (1.2mol) of 2-(2-chloroethoxy)ethanol, add Continue heating and stirring after completion, raise the temperature to 136-140 degrees Celsius, and keep it for 1 hour, and stop heating after TLC shows that the reaction is complete. When the temperature drops to 80 degrees Celsius, add 500 milliliters of 95% ethanol, cool overnight in the refrigerator, filter and recover piperazine dihydrochloride the next day, wash the filter cake fully with a small amount of ethanol, combine the filtrates, and add 30% sodium hydroxide solution 200 grams of alkalization, filter out insoluble inorganic salts; after concentrating to remove the solvent, vacuum distillation collects the fraction at 168-172 degrees Celsius / 10 mmHg to obtain 145 grams of colorless to light yellow transparent liquid 1-[2-( 2-Hydroxyethoxy)ethyl]piperazine, the yield i...
example 3
[0021] Dissolve 100 g (1.2 mol) of anhydrous piperazine in 1 L of toluene, heat to reflux, add dropwise 150 g (1.2 mol) of 2-(2-chloroethoxy)ethanol, and continue to reflux and stir for 5 hours , TLC showed that the reaction was complete and the heating was stopped. When the temperature drops to room temperature, add 200 grams of 30% sodium hydroxide solution to alkalinize, filter out insoluble inorganic salts; after concentrating to remove the solvent, extract the residue with ethyl acetate, pass through dry hydrogen chloride gas, and obtain White solid 1-[2-(2-hydroxyethoxy)ethyl]piperazine hydrochloride; after recrystallization once with 90% ethanol aqueous solution, 180 grams of white crystals can be obtained, the yield is 60%, and the content can reach 99% above;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com