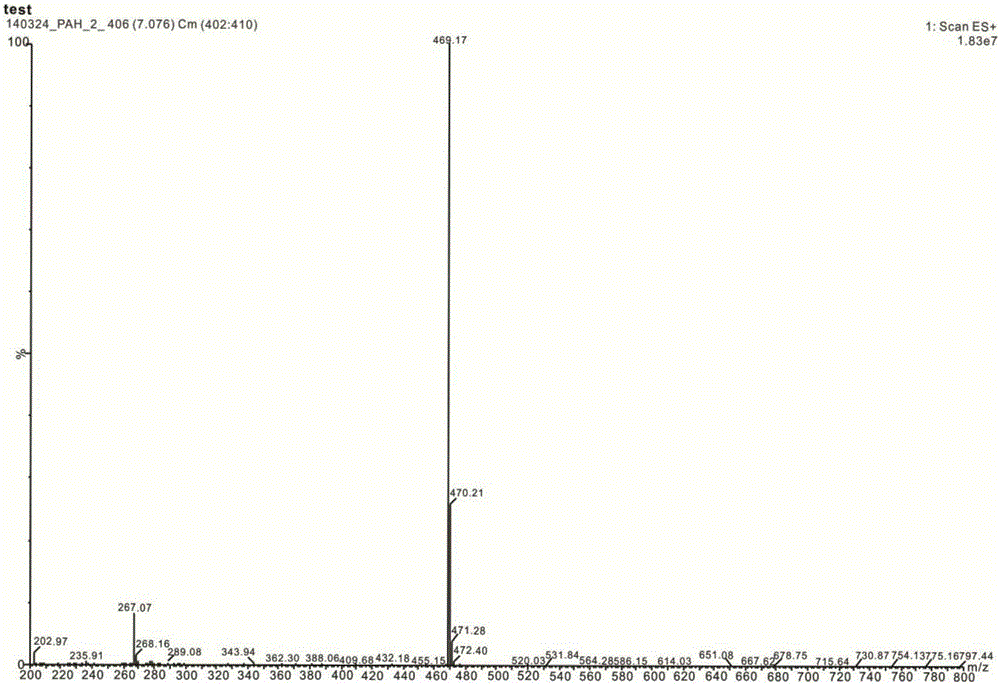
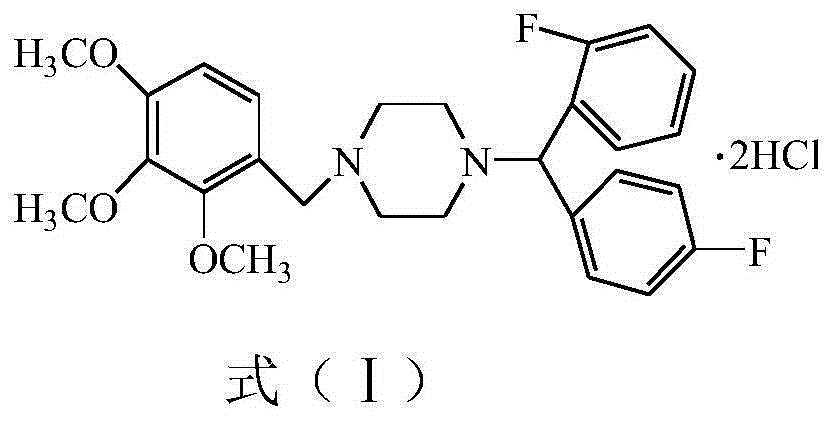
Lomerizine Hydrochloride isomeride and preparation method therefor
A technology of lomerizine hydrochloride isomers and isomers, which is applied in the field of lomerizine hydrochloride isomers and its preparation, can solve the problem of obtaining lomerizine hydrochloride isomers and hydrochloric acid which have not yet been found. Difficult separation of isomers of lomerizine and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
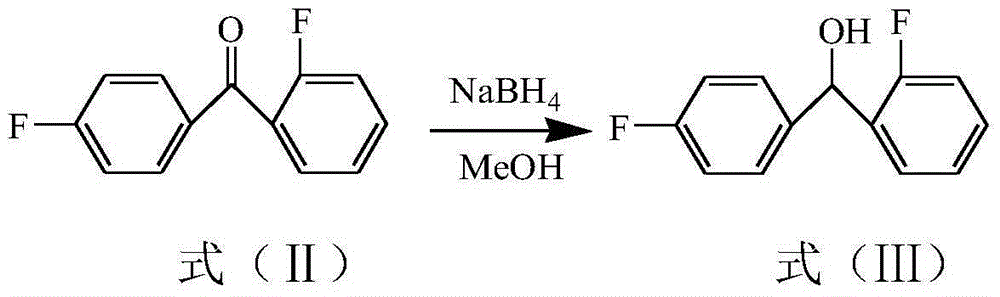
[0037] Add 21.8g of compound II 2,4'-difluorobenzophenone into the flask, then add 50ml of anhydrous methanol and 3.8g of sodium borohydride, and react at -10~-5°C for 5 hours. After the reaction is completed, Add 20ml of dichloromethane to the reaction solution to extract twice, collect the extract, add anhydrous sodium sulfate to the extract and dry for 6 hours, then concentrate under reduced pressure at 40°C to remove the organic solvent, dichloromethane, to obtain 22g of compound III 2,4 '-difluorobenzhydryl alcohol;
[0038] Add 22g of compound III and 8ml of thionyl chloride into the flask, add 1 drop of N,N-dimethylformamide dropwise, and react at 80°C for 6 hours. After the reaction is completed, concentrate under reduced pressure at this temperature to remove Thionyl chloride was obtained to obtain compound IV 2,4'-difluorodiphenylchloromethane; then the obtained compound IV 2,4'-difluorodiphenylchloromethane, 10g benzyltriethylammonium chloride, 7.5g piperazine , 10...
Embodiment 2
[0058] Add 21.8g of compound II 2,4'-difluorobenzophenone into the flask, then add 50ml of absolute ethanol and 5.7g of sodium borohydride, and react at -8~-3°C for 4 hours. After the reaction is completed, Add 20ml of dichloromethane to the reaction solution to extract 3 times, collect the organic phase, add anhydrous sodium sulfate to the organic phase and dry for 8 hours, then concentrate under reduced pressure at 40°C to remove the organic solvent, dichloromethane, to obtain 22.5g of compound III 2 ,4'-difluorobenzhydryl alcohol;
[0059] Add 22.5g of compound III and 8.2ml of thionyl chloride into the flask, drop 1 drop of N,N-dimethylformamide, and react at 80°C for 4 hours. After the reaction is completed, depressurize at this temperature Concentrate to remove thionyl chloride to obtain compound IV 2,4'-difluorodiphenylchloromethane; then the obtained compound IV 2,4'-difluorodiphenylchloromethane, 10g benzyltriethylammonium chloride, 8.2g Add piperazine, 103ml of tolu...
Embodiment 3
[0064] Add 21.8g of compound II 2,4'-difluorobenzophenone to the flask, then add 50ml of absolute ethanol and 4.75g of sodium borohydride, and react at -5 to 0°C for 4 hours. After the reaction is completed, the reaction Add 20ml of dichloromethane to the liquid and extract 3 times, collect the extract, add anhydrous sodium sulfate to the extract and dry for 7 hours, then concentrate under reduced pressure at 50°C to remove the organic solvent dichloromethane to obtain 22.8g of compound III 2,4 '-difluorobenzhydryl alcohol;
[0065] Add 22.8g of compound III and 8.2ml of thionyl chloride into the flask, drop 1 drop of N,N-dimethylformamide, and react at 70°C for 6 hours. After the reaction is completed, decompress at this temperature Concentrate to remove thionyl chloride to obtain compound IV 2,4'-difluorodiphenylchloromethane; then the obtained compound IV 2,4'-difluorodiphenylchloromethane, 10g benzyltriethylammonium chloride, 8.3g Add piperazine, 110ml of toluene and 100m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com