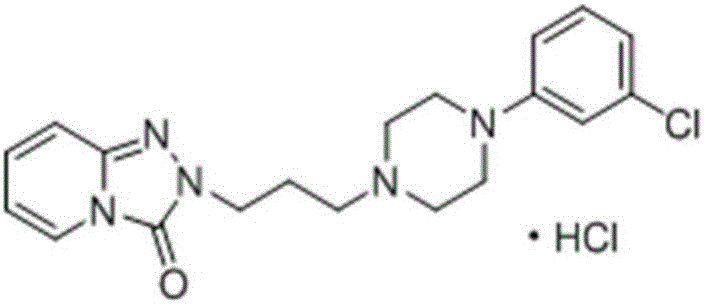
Preparation method of trazodone hydrochloride
A technology of trazodone hydrochloride and hydrochloride, applied in the field of preparation of trazodone hydrochloride, can solve problems such as inability to completely convert trazodone, and achieve the effects of reducing production cost, improving total yield and stabilizing process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1 The present invention adds sodium hydroxide solution to prepare trazodone
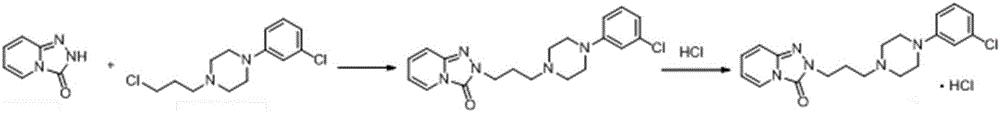
[0043] Into a 2L three-necked flask at room temperature, add 100g (0.323mol) N-(3-chloro-phenyl)-N'-(3-chloropropyl)-piperazine hydrochloride, 52.4g (0.388mol) pyridine Triadimefon, 1500mL isopropanol, start mechanical stirring, add 29.7g (0.743mol) sodium hydroxide, nitrogen protection, heat up to reflux, react for 26 hours, HPLC detects N-(3-chloro-phenyl)-N' The -(3-chloropropyl)-piperazine content was 0.02%. Control the temperature to 60-70°C for hot filtration, transfer the filtrate into a reaction flask, add 120mL of 10% sodium hydroxide aqueous solution, stir and heat up to reflux for 8 hours, cool down to 5°C for crystallization, keep stirring for 2 hours, filter, and vacuum dry at 50°C 110 g (0.296 mol) of trazodone was obtained, with a yield of 92%.
Embodiment 2
[0044] Embodiment 2 The present invention adds potassium hydroxide solution to prepare trazodone
[0045] Into a 2L three-necked flask at room temperature, add 100g (0.323mol) N-(3-chloro-phenyl)-N'-(3-chloropropyl)-piperazine hydrochloride, 52.4g (0.388mol) pyridine Triadimefon, 1500mL isopropanol, start mechanical stirring, add 29.7g (0.743mol) sodium hydroxide, nitrogen protection, heat up to reflux, react for 26 hours, N-(3-chloro-phenyl)-N The '-(3-chloropropyl)-piperazine content was 0.02%. Control the temperature to 60-70°C for hot filtration, transfer the filtrate to a reaction flask, add 120mL of 10% potassium hydroxide aqueous solution, stir and heat up to reflux for 8 hours, cool down to 5°C for crystallization, keep stirring for 2 hours, filter, and vacuum dry at 50°C 108 g (0.290 mol) of trazodone was obtained, with a yield of 90%.
Embodiment 3
[0046] Embodiment 3 uses the trazodone prepared by the present invention to synthesize trazodone hydrochloride
[0047] Into a 500mL three-necked flask at room temperature, add 50g (0.134mol) trazodone and 300mL ethanol in sequence, start mechanical stirring, heat up to 60°C, after the solid dissolves, add 12mol / L HCl aqueous solution dropwise, adjust the pH of the system to 3, and then slowly The temperature was lowered to 0°C, a large amount of white solid was precipitated, kept stirring for 2 hours, filtered, and vacuum-dried at 50°C to obtain 51 g (0.125 mol) of trazodone hydrochloride with a yield of 93%.
[0048] The residual amount of impurity N-(3-chloro-phenyl)-N'-(3-chloropropyl)-piperazine in Comparative Example 1 and Examples 1-3 was detected respectively. The detection method and process used are:
[0049] 1.1 Instruments and equipment, as shown in Table 1 below.
[0050] Table 1
[0051]
[0052] 1.2 Solution preparation
[0053] Standard solution (0.025μg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com