Method for preparing 3-(4-chlorobutyryl)-1H-indole-5-methylcyanogen
A technology of chlorobutyryl and indole is applied in the field of preparation of 3--1H-indole-5-methyl cyanide, and can solve the problems of insolubility, difficulty in stirring, difficulty in mass preparation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
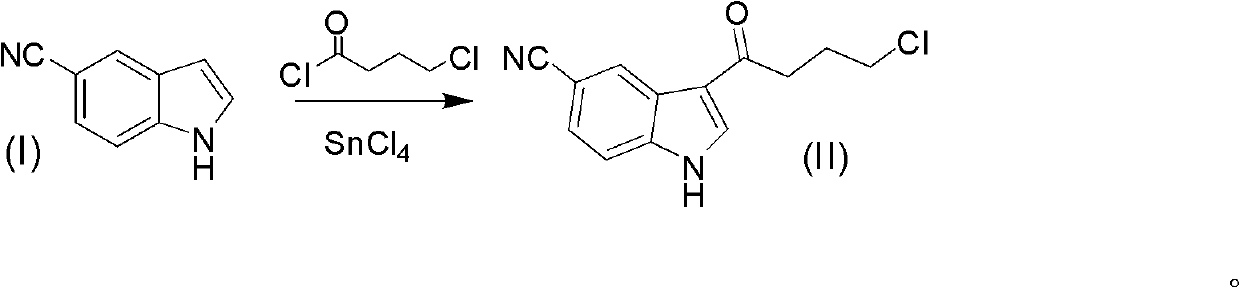
[0035] 3-(4-chlorobutyryl)-1H-indole-5-cyanide (II) preparation method one
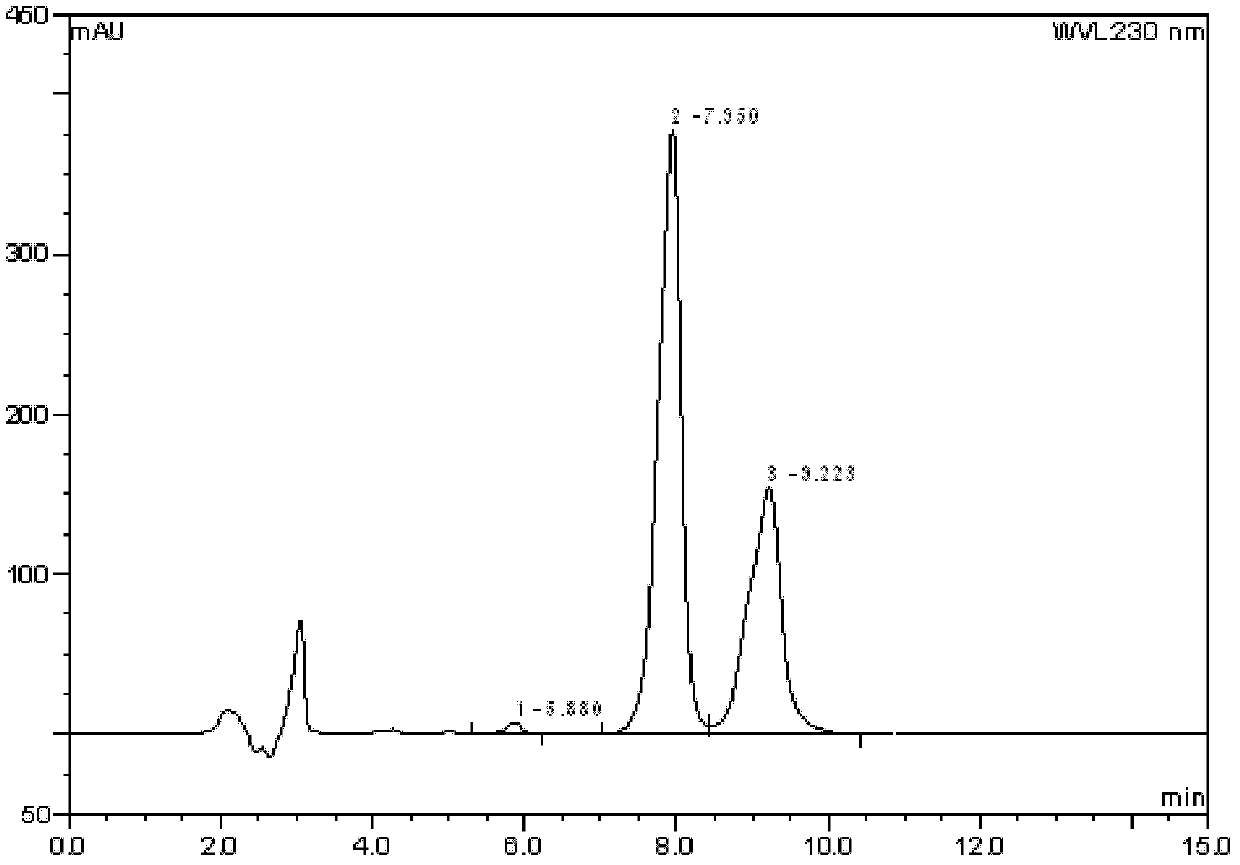
[0036] 1H-indole-5-carbonitrile (1.00mol, 142g) was dissolved in 1300ml (1716g) of dichloromethane, stirred until dissolved, under ice bath, the temperature of the system was lowered to 0°C, and tin tetrachloride ( 1.05mol, 274g), the temperature control system is 0-5°C, after dropping, stir for 0.5 hours, add nitromethane 900ml (1017g), add tetrachlorobutyryl chloride (1.00mol, 141g) dropwise after 10min, and react for 1 hour . The reaction solution was poured into 3000ml of ice-water mixture and stirred for 0.5 hours. Add 5000ml of ethyl acetate, extract, adjust the pH of the organic phase to neutral with saturated aqueous sodium carbonate solution, separate the liquids, wash the organic phase with saturated brine, concentrate and wash with 500ml of ethyl acetate to obtain 180.6g of white crystals, with a yield of 73 %. Melting point: 186-189°C.
[0037] ESI-MS[M+H]+: 247.06
[0038] 1 H-NMR (...
Embodiment 2
[0040] 3-(4-chlorobutyryl)-1H-indole-5-cyanide (II) preparation method two
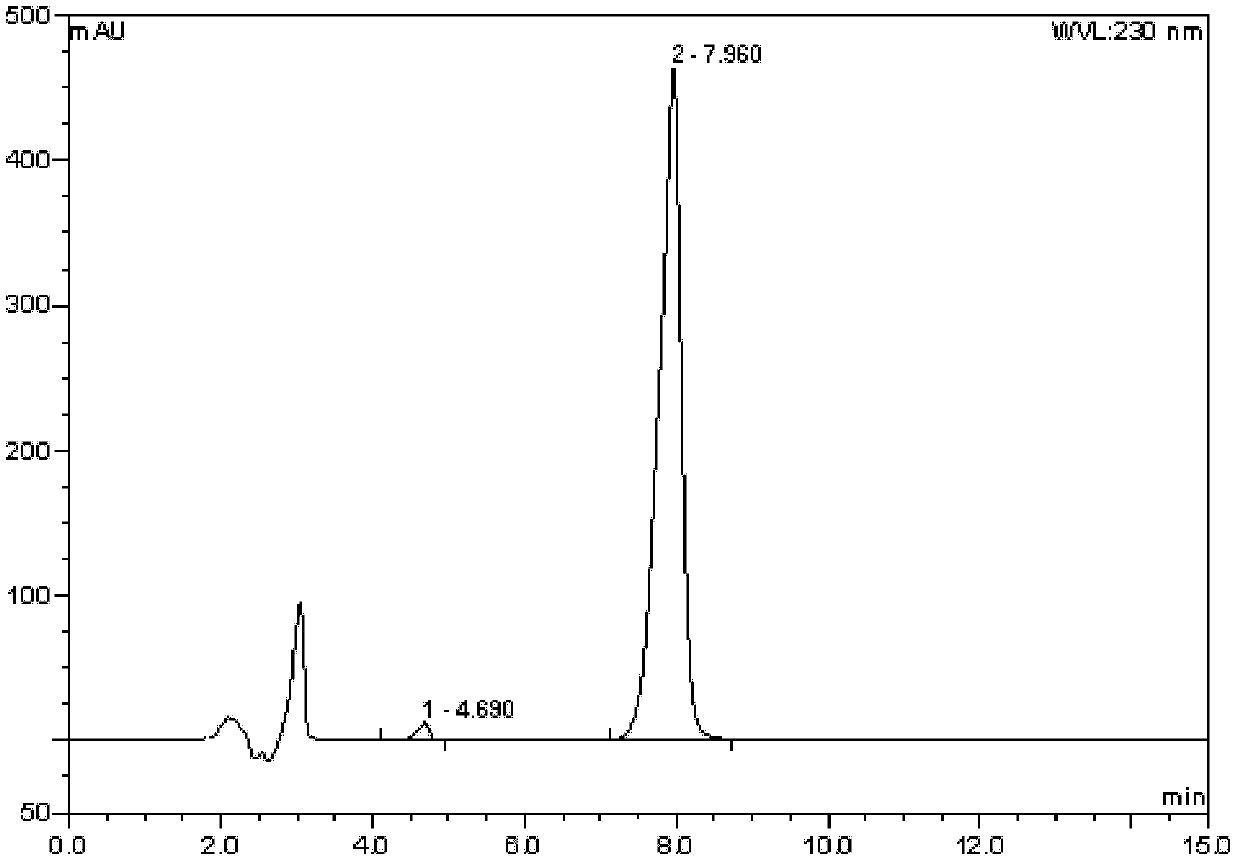
[0041] 1H-indole-5-carbonitrile (1.00mol, 142g) was dissolved in a mixed solvent of 2000ml (2640g) of dichloromethane and 1000ml (1132g) of nitromethane, and the temperature of the system was lowered to 0°C under an ice bath, Tin tetrachloride (1.05mol, 274g) was added dropwise, and the temperature of the system was controlled at 0-5°C. Continue stirring for 0.5 hours after dropping, add tin tetrachloride (1.00mol, 141g) dropwise, react for 0.8 hours, pour the reaction solution into 3000ml ice-water mixture, and stir for 0.5 hours. Add 5000ml of ethyl acetate, extract, adjust the pH of the organic phase to neutral with a saturated sodium carbonate aqueous solution, and separate the layers. The organic phase is washed with saturated brine, concentrated and washed with ethyl acetate to obtain 172.7 g of white crystals, with a yield of 70%.
[0042] ESI-MS[M+H]+: 247.06
Embodiment 3
[0044] 3-(4-chlorobutyryl)-1H-indole-5-cyanide (II) preparation method three
[0045] Dissolve 1H-indole-5-carbonitrile (1.00mol, 142g) in 2000ml (2640g) of dichloromethane, stir until completely dissolved, lower the temperature of the system to -20°C under cooling pump circulation, add tetrachloride dropwise Tin (110mol, 286g), the temperature control system is -10℃~-5℃. Continue to stir for 0.5 hours after dropping, add 1200ml (1260g) of nitroethane, dropwise add tetrachlorobutyryl chloride (1mol, 141g) after 10min, react for 1.5 hours, pour the reaction solution into 3000ml of ice-water mixture, and stir for 0.5 hours . Add 5000ml of ethyl acetate, extract, adjust the pH of the organic phase to neutral with a saturated sodium carbonate aqueous solution, separate the liquids, wash the organic phase with saturated brine, concentrate and wash with 500ml of ethyl acetate to obtain 200.2g of white crystals, with a yield of 81 %.
[0046] ESI-MS[M+H]+: 247.06
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com