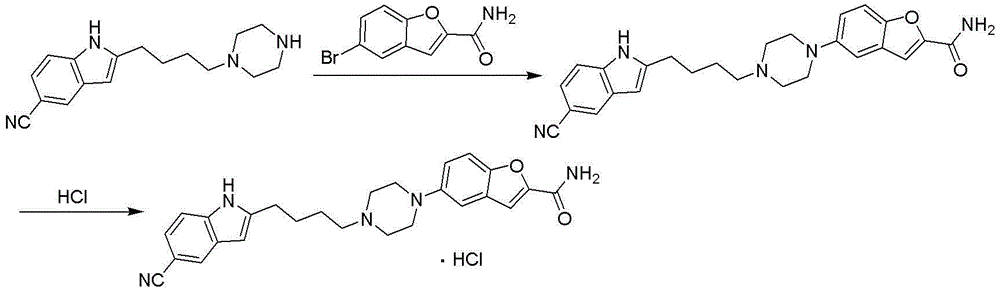
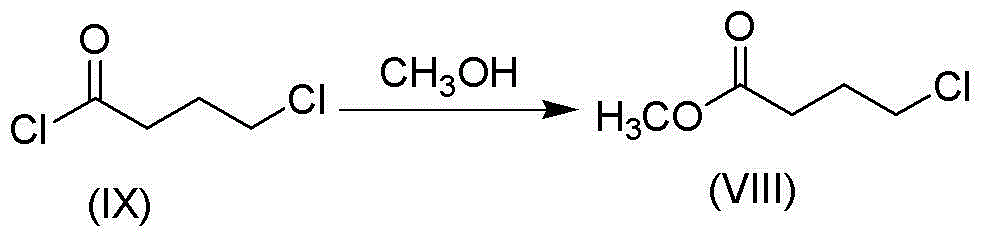
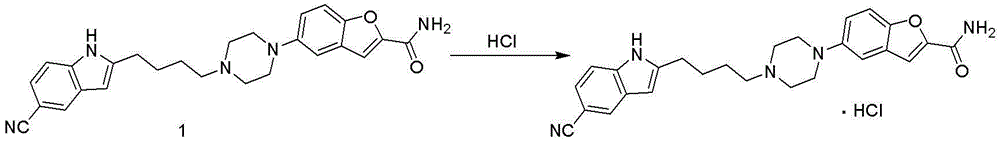
Method for preparing vilazodone or hydrochloride thereof
A kind of technology of vilazodone and compound, applied in the field of preparing vilazodone or its hydrochloride, can solve problems such as low reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Preparation of methyl 4-chlorobutyrate
[0080] Take 49.8g of potassium carbonate in 400ml of methanol, cool to 0-5°C with an ice-water bath, add 42.3g of 4-chlorobutyryl chloride dropwise, heat to reflux for 4 hours after the addition, then filter, and distill the filtrate under reduced pressure to obtain 41.2g of crude product , used directly in the next reaction.
[0081] ESI-MS[M+H] + :137.04
[0082] 1 H-NMR (CDCl 3 ): δ1.92-1.96 (m, 2H), 2.31 (t, 2H), 3.30 (t, 2H), 3.68 (s, 3H).
Embodiment 2
[0084] Preparation of 4-bis(2-hydroxyethyl)aminobutyric acid methyl ester
[0085] The 41.2g crude product of methyl 4-chlorobutyrate prepared by the previous step reaction was dissolved in 400ml of acetonitrile, 41.5g of potassium carbonate and 31.5g of bis(2-hydroxyethyl)amine were added, heated to reflux for 12 hours, filtered after cooling, and the filtrate was reduced Distill under pressure, add 400ml isopropanol to the residue, slowly add 30ml (37%) concentrated hydrochloric acid at room temperature, filter after the precipitation is complete, dissolve the filter cake in 200ml water, extract with 3*200ml ethyl acetate, and use anhydrous sulfuric acid for the organic phase It was dried over sodium, filtered and distilled under reduced pressure to obtain 23.4 g of product.
[0086] ESI-MS[M+H] + :206.14
[0087] 1 H-NMR (CDCl 3 ): δ1.68-1.72 (m, 2H), 2.20 (t, 2H), 2.34 (t, 2H), 2.52 (t, 4H), 3.56 (t, 4H), 3.68 (s, 3H).
Embodiment 3
[0089] Preparation of 4-bis(2-chloroethyl)aminobutyric acid methyl ester
[0090] Take 20.5g of 4-bis(2-hydroxyethyl)aminobutyric acid methyl ester and 30.0g of pyridine and dissolve it in 320ml of tetrahydrofuran, cool to 0-5°C, add 27.5g of phosphorus trichloride dropwise, after the addition, heat Reflux for 4 hours, filter after cooling, distill the filtrate under reduced pressure, add 200ml ethyl acetate and 100ml ice water to the residue, adjust the pH to about 7 with 1N sodium hydroxide solution, separate the liquids, dry the organic phase with anhydrous sodium sulfate and filter , The filtrate was evaporated to dryness under reduced pressure to obtain 17.4 g of product.
[0091] ESI-MS[M+H] + :242.07
[0092] 1 H-NMR (CDCl 3 ): δ1.71-1.76 (m, 2H), 2.22 (t, 2H), 2.33 (t, 2H), 2.53 (t, 4H), 3.55 (t, 4H), 3.69 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com