Preparation method of vilazodone hydrochloride
A technology of vilazodone hydrochloride and formyl nitrile, which is applied in the field of drug synthesis, can solve the problems of high preparation cost, and achieve the effects of stable product quality, shortened reaction steps, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
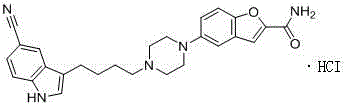
[0049] Embodiment 1: the synthesis of vilazodone hydrochloride
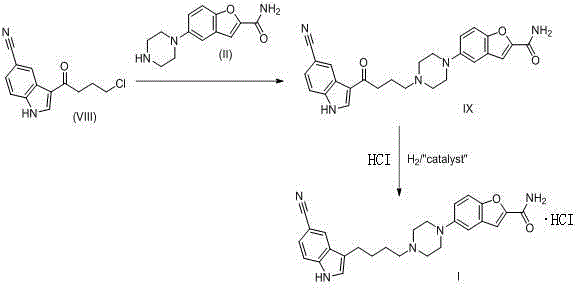
[0050] 3-(4-Chlorobutyryl)-1H-indole-5-carbonitrile (VIII) (95g, 0.41mol, 1.0eq), 5-(piperazin-1-yl)benzofuran-2-carboxamide (II) (100g, 0.41mol.1.0eq), KI (6.8g, 0.041mol, 0.1eq), TEA (226ml, 4.0eq) and DMF (1L) were added to a 2L three-necked flask, and vacuum degassed under stirring 2min, nitrogen replacement, so 3 times. The reaction solution was stirred at room temperature for 1 hour, then heated to an internal temperature of 85-90°C, and reacted for 48 hours. The remaining material VIII) was monitored by HPLC to be less than 3%, and the heating was stopped and cooled to room temperature. 1.1 L of water was added dropwise to the reaction liquid until the white solid was completely precipitated, which took about 5 hours, filtered, and the filter cake was beaten with 2 L of water for 1 hour, filtered, and vacuum-dried to obtain 168 g of crude product. 168g of the crude product was added to 3L of absolute eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com