Orally disintegrating tablet containing vilazodone hydrochloride solid dispersions and preparation method thereof
A technology of vilazodone hydrochloride and solid dispersion, which is applied in the field of pharmaceutical preparations, can solve the problems of poor dissolution and low solubility of preparations, and achieve the effect of improving dissolution and drug solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
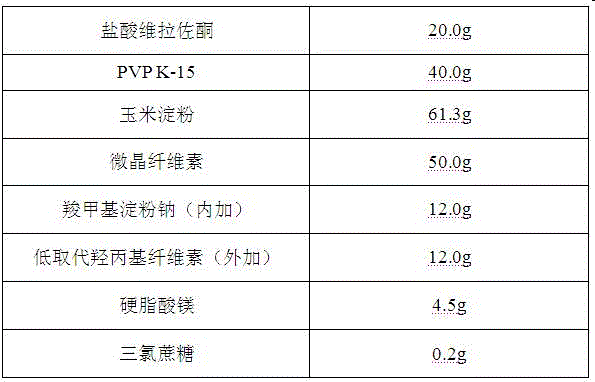
[0021] Vilazodone hydrochloride orally disintegrating tablets are prepared from the following components, in the amount of 1000 tablets
[0022]
[0023] Its preparation method comprises the following steps:
[0024] (1) Take vilazodone hydrochloride, dissolve it in 10 times the weight of ethanol solution, add the prescribed amount of PEG6000, stir to dissolve into a clear and transparent solution, and remove the solvent by rotary evaporation to obtain a solid dispersion.
[0025] (2) The obtained solid dispersion is pretreated through a 60-mesh sieve.
[0026] (3) Uniformly mix the pretreated vilazodone hydrochloride solid dispersion with the prescribed amount of lactose, microcrystalline cellulose, and internally added croscarmellose sodium, then dry granulate, add externally added croscarmellose Sodium cellulose, magnesium stearate and acesulfame K are evenly mixed and compressed into tablets.
[0027] test results:
[0028] The disintegration time is 25s, compared wi...
Embodiment 2
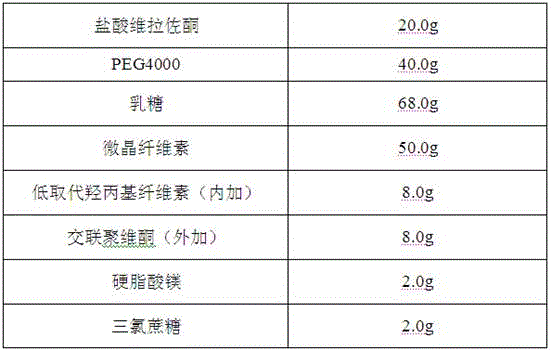
[0030] Vilazodone hydrochloride orally disintegrating tablets are prepared from the following components, in the amount of 1000 tablets
[0031]
[0032] Its preparation method comprises the following steps:
[0033] (1) Take vilazodone hydrochloride, dissolve it in 50 times the weight of ethanol solution, add the prescribed amount of PVPK-30, stir to dissolve it into a clear and transparent solution, and remove the solvent by rotary evaporation to obtain a solid dispersion.
[0034] (2) The obtained solid dispersion is pretreated through a 120-mesh sieve.
[0035] (3) Uniformly mix the pretreated vilazodone hydrochloride solid dispersion with the prescribed amount of mannitol, microcrystalline cellulose, and internally added low-substituted hydroxypropyl cellulose, then dry granulate, add externally added low-substituted hydroxypropyl cellulose Base cellulose, magnesium stearate and aspartame are evenly mixed and compressed into tablets.
[0036] test results:
[0037] ...
Embodiment 3
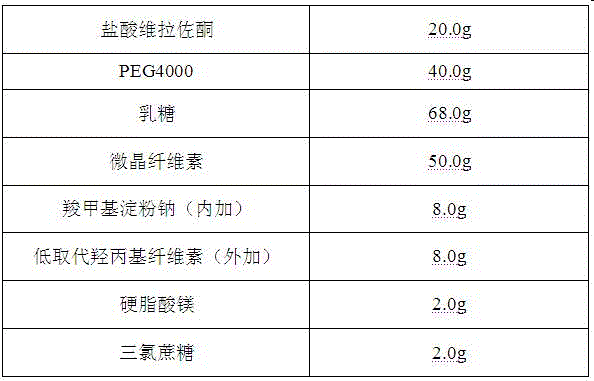
[0039] Vilazodone hydrochloride orally disintegrating tablets are prepared from the following components, in the amount of 1000 tablets
[0040]
[0041] Its preparation method comprises the following steps:
[0042] (1) Take vilazodone hydrochloride, dissolve it in 25 times the weight of ethanol solution, add the prescribed amount of PVPK-90, stir to dissolve into a clear and transparent solution, and remove the solvent by rotary evaporation to obtain a solid dispersion.
[0043] (2) The obtained solid dispersion is pretreated through a 120-mesh sieve.
[0044] (3) Uniformly mix the pretreated vilazodone hydrochloride solid dispersion with the prescribed amount of mannitol, pregelatinized starch, and internally added crospovidone, then dry granulate, add externally added low-substituted hydroxypropyl fiber Sodium stearyl fumarate and sucralose were evenly mixed and compressed into tablets.
[0045] test results:
[0046] The disintegration time is 23s, compared with the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com