Preparation method and application of vilazodone hydrochloride IV crystal
A technology of hydrochloric acid and hydrochloride, applied in the direction of organic chemistry, etc., can solve the problems of unexplained crystal form preparation temperature range, complex preparation process of IV crystal form, large amount of solvent, etc., to achieve stable process scale-up, easy industrial production, Solve the effect of easy crystal mixing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 (20120718-C10)
[0058] At room temperature, tetrahydrofuran (25.0ml) was added to a three-necked flask, and 5-(4-(4-(5-cyano-3-indolyl)butyl)-1-piperazinyl)benzofuran was added under stirring -2-Carboxamide (1.01g, 2.29mmol), stirred at the same temperature until dissolved, raised the temperature to 40-55°C, slowly added concentrated hydrochloric acid (0.25g) dropwise, after the dropwise completion, kept stirring for 2 hours, filtered to obtain The white solid wet product was put into a vacuum oven at 85-90°C and dried under reduced pressure to constant weight to obtain a dry product (0.97g, molar yield 88.6%), and its X-ray diffraction pattern is shown in Figure 5 .
Embodiment 2
[0059] Example 2 (20120719-C10)
[0060] At room temperature, tetrahydrofuran (300.0ml) was added to a three-necked flask, and 5-(4-(4-(5-cyano-3-indolyl)butyl)-1-piperazinyl)benzofuran was added under stirring -2-Carboxamide (10.08g, 22.83mmol), stirred at the same temperature until dissolved, raised the temperature to 50-55°C, slowly added concentrated hydrochloric acid (2.32g), dripped, kept stirring for 2 hours, filtered, and obtained The white solid wet product was put into a vacuum oven at 130-135°C and dried under reduced pressure to constant weight to obtain a dry product (9.53g, molar yield 87.3%), and its X-ray diffraction pattern is shown in Image 6 .
Embodiment 3
[0061] Example Three (WL-120720)
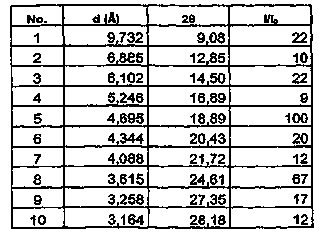
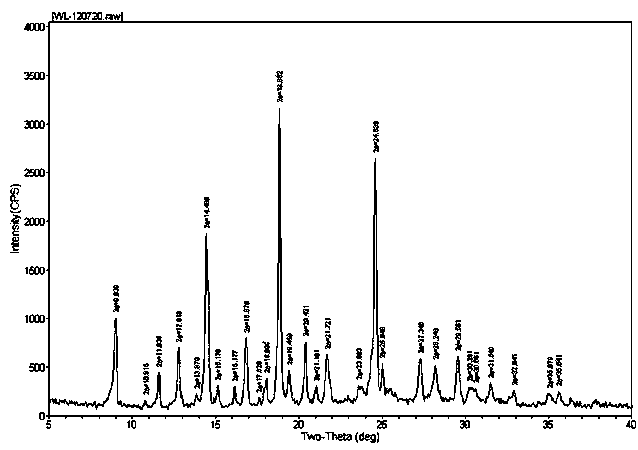
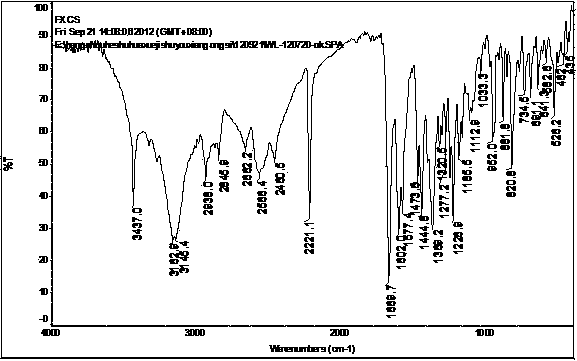
[0062] At room temperature, tetrahydrofuran (2500ml) was added into a three-necked flask, and 5-(4-(4-(5-cyano-3-indolyl)butyl)-1-piperazinyl)benzofuran was added under stirring- 2-Carboxamide (100.05g, 0.227mol), stirred at the same temperature until dissolved, then raised the temperature to 40-55°C, slowly added concentrated hydrochloric acid (22.2g) dropwise, and kept stirring for 2 hours, then filtered to obtain a white The solid wet product was put into a vacuum oven at 90-95°C and dried under reduced pressure to constant weight to obtain a dry product (94.0g, molar yield 86.8%), its X-ray diffraction pattern, IR absorption spectrum and Raman absorption spectrum See you separately Figure 7 , Figure 8 and Figure 9 .
[0063] The above preparation method, as a key step, can be used to prepare vilazodone and pharmaceutically acceptable salts thereof.
[0064] The above-mentioned preparation method serves as a key step in the manufac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com