Crystalline forms of vilazodone hydrochloride and vilazodone free base
a technology of vilazodone and hydrochloride, which is applied in the field of crystalline forms of vilazodone hydrochloride and vilazodone free base, can solve the problems of no “standard", the inability to predict the number of polymorphic forms of a given compound, and the inability to achieve the effect of increasing the number of polymorphic forms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Crystalline Form B of Vilazodone Hydrochloride
example 1a
[0383]Vilazodone free base (118 g) and methanol (2360 mL) were charged into a round bottom flask at ambient temperature. The resulting mixture was cooled to 4° C. and 10% w / w methanolic hydrochloric acid solution (236 mL) was slowly added, stirred the resulting solution at 0-5° C. for 30 minutes. The solid was collected by filtration and dried at 45-50° C. for 3.5 hours to afford the title compound.
[0384]Yield: 107.6 g;
[0385]Water Content: 8.46% w / w as measured by Karl Fischer method.
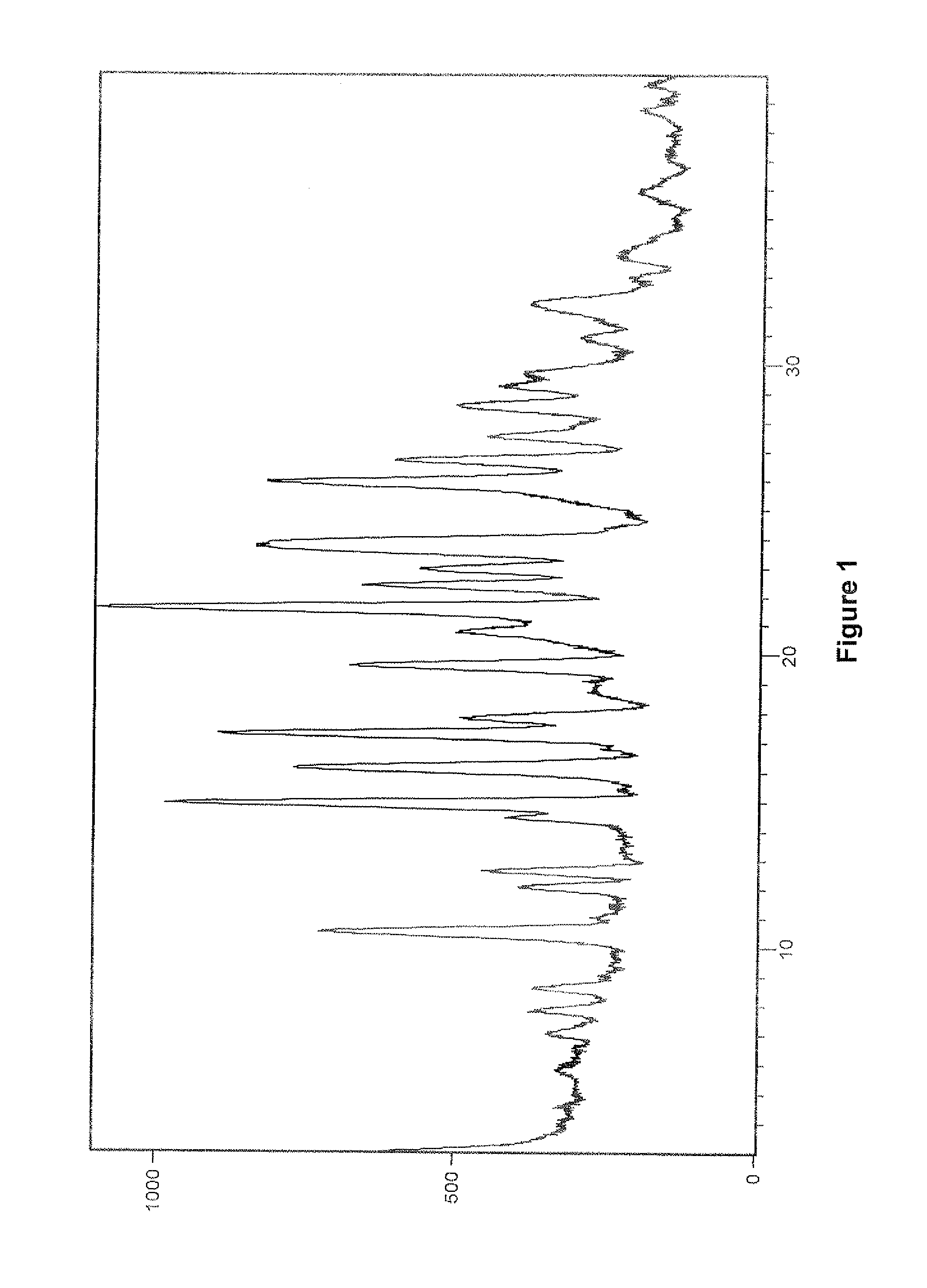
[0386]The PXRD pattern of crystalline Form B of vilazodone hydrochloride obtained is in accordance with FIG. 1.
example 1b
[0387]Vilazodone free base (1.5 g) and methanol (30 mL) were charged into a round bottom flask at −10° C. 10% w / w Methanolic hydrochloric acid solution (3 mL) was slowly added to the resulting mixture at −10° C. and further stirred for 30 minutes. The solid was collected by filtration and dried at 45-50° C. for 30 minutes to afford the title compound.
[0388]Yield: 1.4 g
[0389]Water Content: 8.46% w / w as measured by Karl Fischer method.
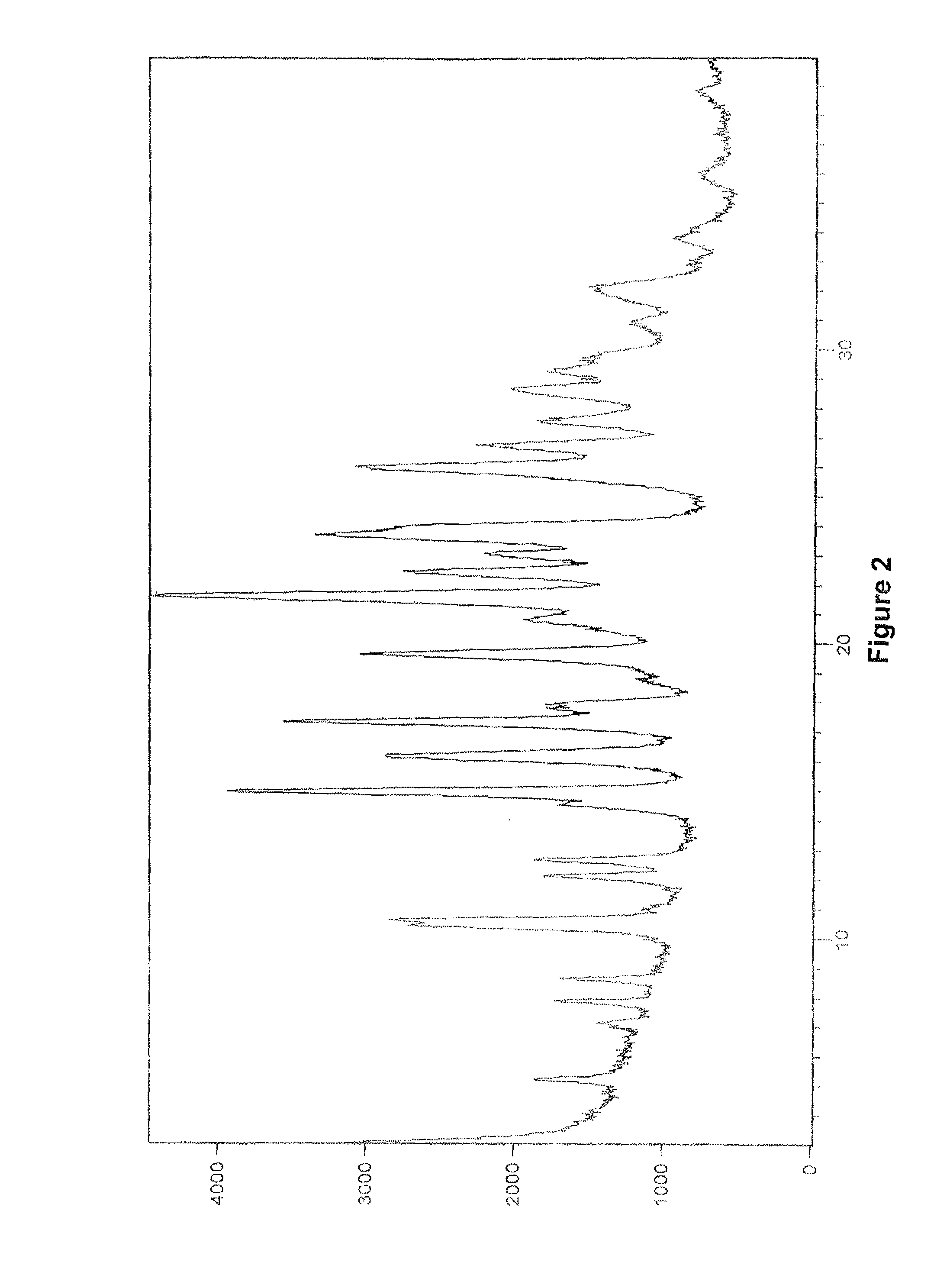
[0390]The PXRD pattern of crystalline Form B of vilazodone hydrochloride obtained is in accordance with FIG. 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Angle | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com