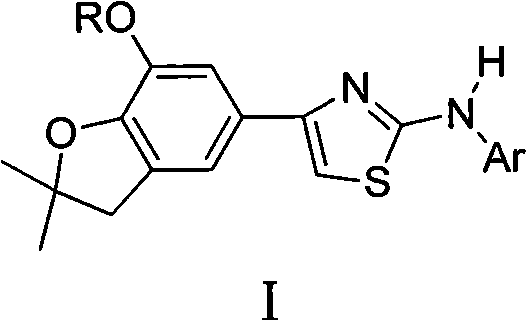
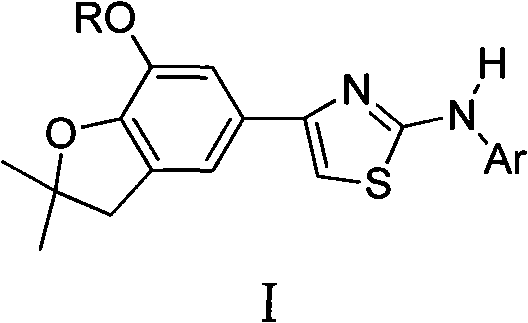
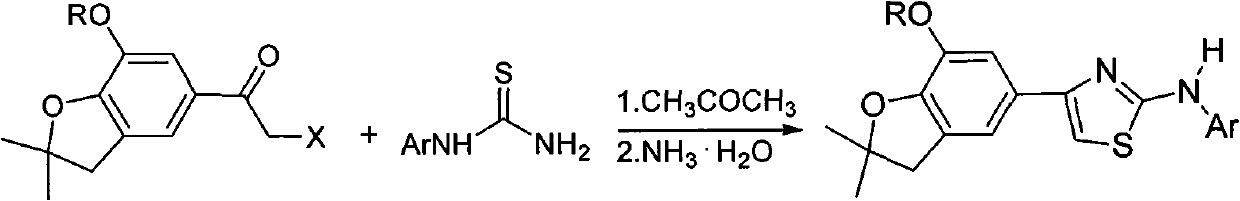4-(benzofuran-5-yl)-2-aromatic aminothiazole and preparation method and application thereof
A technology of arylaminothiazole and arylaminothiazole salt, which is applied in the field of new compounds and their preparation, and can solve the problems of no research and development reports on application and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0021]
[0022] (1) Preparation of 2-bromo-1-(7-methoxy-2,2-dimethyl-2,3-dihydrobenzofuran-5-yl)ethanone
[0023] 0.02mol 1-(7-methoxy-2,2-dimethyl-2,3-dihydrobenzofuran-5-yl)ethanone, 80mL ethanol, stirring and reflux, add 0.04mol copper bromide in batches , reacted for 2h, filtered the reaction solution while it was hot, recovered the solvent by distillation, dissolved ethyl acetate, washed with dilute acid, filtered, washed the filtrate with water, separated, dried, and recrystallized from ethanol to obtain 2-bromo-1-(7-methoxy -2,2-Dimethyl-2,3-dihydrobenzofuran-5-yl)ethanone, yield 50.0%, melting point 90-91°C.
[0024] 2) Preparation of 4-(7-methoxy-2,2-dimethyl-2,3-dihydrobenzofuran-5-yl)-2-anilinothiazole
[0025] 2.0mmol 2-bromo-1-(7-methoxy-2,2-dimethyl-2,3-dihydrobenzofuran-5-yl)ethanone, 1.8mmol phenylthiourea, 20mL acetone, Heat and stir, react for 2 hours, the reaction solution is cooled to precipitate solid, filtered, and dried to obtain 4-(7-methoxy-2,2-...
Embodiment 24
[0027]
[0028] According to the method of Example 1, 2-bromo-1-(7-methoxy-2,2-dimethyl-2,3-dihydrobenzofuran-5-yl)ethanone and 2-tolylthio Urea reacted for 1.0h to obtain 4-(7-methoxy-2,2-dimethyl-2,3-dihydrobenzofuran-5-yl)-2-(2-methylanilino)thiazole , the yield is 92.4%, and the melting point is 146.5-147.5°C. 1 H NMR (CDCl 3 , 300MHz), δ: 1.53(s, 6H, 2×CH 3 ), 2.35(s, 3H, CH 3 ), 3.06 (s, 2H, CH 2 ), 3.95(s, 3H, OCH 3), 6.59 (s, 1H, thiazole ring 5-H), 7.10 (m, 2H, C 6 h 2 ), 7.24~7.62 (m, 4H, C 6 h 4 ); EI-MS (m / z): 367.2 (M + ).
Embodiment 34
[0030]
[0031] According to the method of Example 1, 2-bromo-1-(7-methoxy-2,2-dimethyl-2,3-dihydrobenzofuran-5-yl)ethanone and 3-methylbenzene thiourea reaction for 2.0h to obtain 4-(7-methoxy-2,2-dimethyl-2,3-dihydrobenzofuran-5-yl)-2-(3-methylanilino ) Thiazole, the yield is 87.8%, and the melting point is 177.9~178.5°C. 1 H NMR (CDCl 3 , 300MHz), δ: 1.43(s, 6H, 2×CH 3 ), 2.31 (s, 3H, CH 3 ), 3.05(s, 2H, CH 2 ), 3.83 (s, 3H, OCH 3 ), 6.77~7.58 (m, 7H, C 6 h 4 , C 6 h 2 , thiazole ring 5-H), 10.14 (s, 1H, NH); EI-MS (m / z): 367.2 (M + ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com