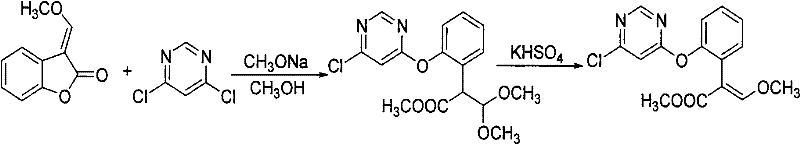
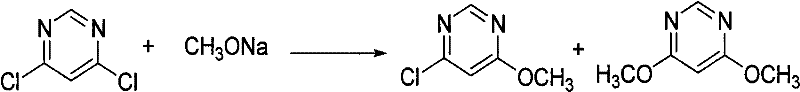
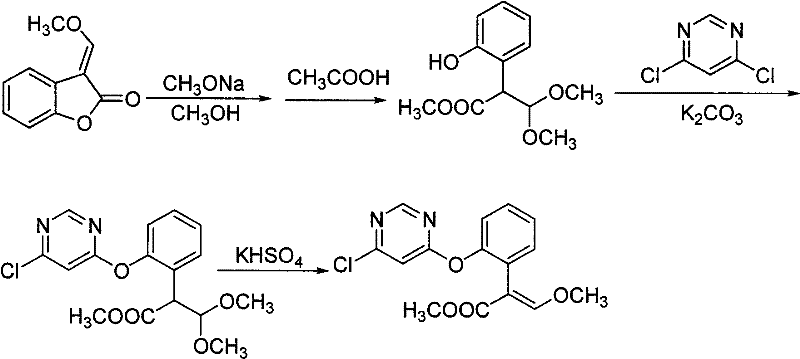
Synthetic method of azoxystrobin and special intermediate for synthesis
A technology of chloropyrimidine and reaction time, which is applied in the field of synthesis technology of chemical substances, can solve the problems of low yield, complicated operation, consumption and the like, and achieves the effects of high yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The steps are:
[0044] a: Mix 0.1mol of the raw material 3-(α-methoxy)methylenebenzofuran-2-(3H)-one with 0.05mol of potassium carbonate in 80mL of toluene solvent, cool the temperature to 0°C, and add 28% Sodium methoxide methanol solution 21.2g (containing 0.11mol of sodium methoxide), reacted for 0.4 hours;
[0045] b: Add 0.11 mol of 4,6-dichloropyrimidine and 1.7 g of catalyst DABCO to the reaction solution in the previous step, react for 1 hour, remove inorganic salts by filtration, wash the filtrate with water, and recover toluene by distillation;
[0046] c: Add 1.5 g of catalyst potassium bisulfate to the distillation residue in the upward step, heat to 132° C. for 1.5 hours at -0.05 MPa, and then cool down.
[0047] d: Dissolve the previous reaction mixture with toluene, wash with water, recover the toluene solvent, then dissolve the crude product with butyl acetate, recrystallize and filter to obtain (E)-2-[2-(6-chloropyrimidin-4-yloxy) Phenyl]-3-methoxyme...
Embodiment 2
[0049] The steps are:
[0050] a: Mix 0.1 mol of the raw material 3-(α-methoxy)methylenebenzofuran-2-(3H)-one with 0.1 mol of potassium carbonate in 80 mL of toluene solvent, cool the temperature to 5°C, and add 28% 21.2 g of sodium methoxide methanol solution (containing 0.11 mol of sodium methoxide), reacted for 0.5 hours;
[0051] b: Add 0.11mol of 4,6-dichloropyrimidine and 1.7g of catalyst DABCO to the reaction solution in the previous step, react for 1.2h, filter to remove inorganic salts, wash the filtrate with water, and recover toluene by distillation;
[0052] c: Add 1.5 g of catalyst potassium bisulfate to the distillation residue of the upward step reaction, and heat to 138° C. to react at -0.04 MPa, until the coupling product content in the reaction system detected by HPLC is 0.85%, which is the end point of the reaction;
[0053]d: Dissolve the previous reaction mixture with toluene, wash with water, recover the toluene solvent, then dissolve the crude product w...
Embodiment 3
[0055] The steps are:
[0056] a: Mix 0.1 mol of the raw material 3-(α-methoxy)methylenebenzofuran-2-(3H)-one with 0.1 mol of potassium carbonate in 100 mL of toluene solvent, cool at 8°C, and add sodium methoxide successively 0.11mol, methanol 10mL, react for 0.5 hours;
[0057] b: Add 0.11mol of 4,6-dichloropyrimidine and 2.0g of catalyst DABCO to the reaction solution in the previous step, react for 1.5h, filter to remove inorganic salts, wash the filtrate with water, and recover toluene by distillation;
[0058] c: Add 1.5 g of catalyst potassium bisulfate to the distillation residue of the upward step reaction, and heat to 140° C. to react at -0.03 MPa until the coupling product content in the reaction system detected by HPLC is 0.9% as the end point of the reaction;
[0059] d: Dissolve the reaction system with toluene, wash with water, recover the toluene solvent, then dissolve the crude product with butyl acetate, recrystallize and filter to obtain (E)-2-[2-(6-chlorop...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com