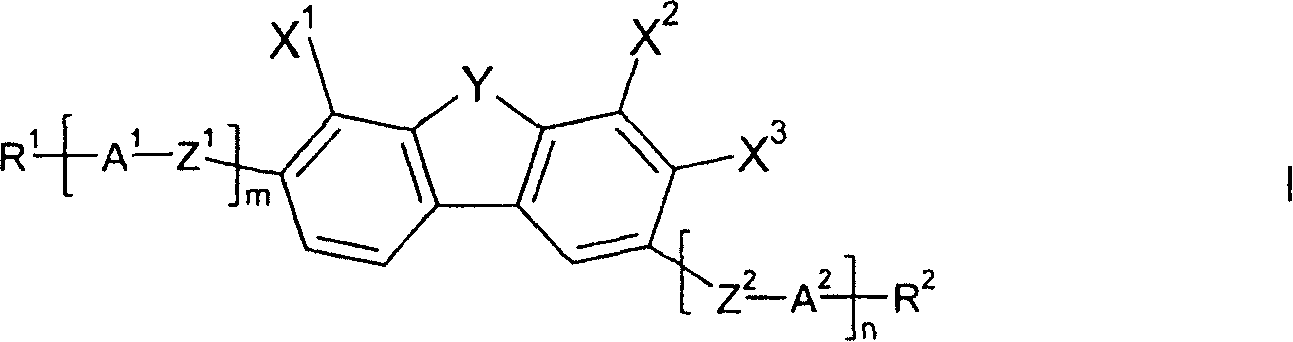
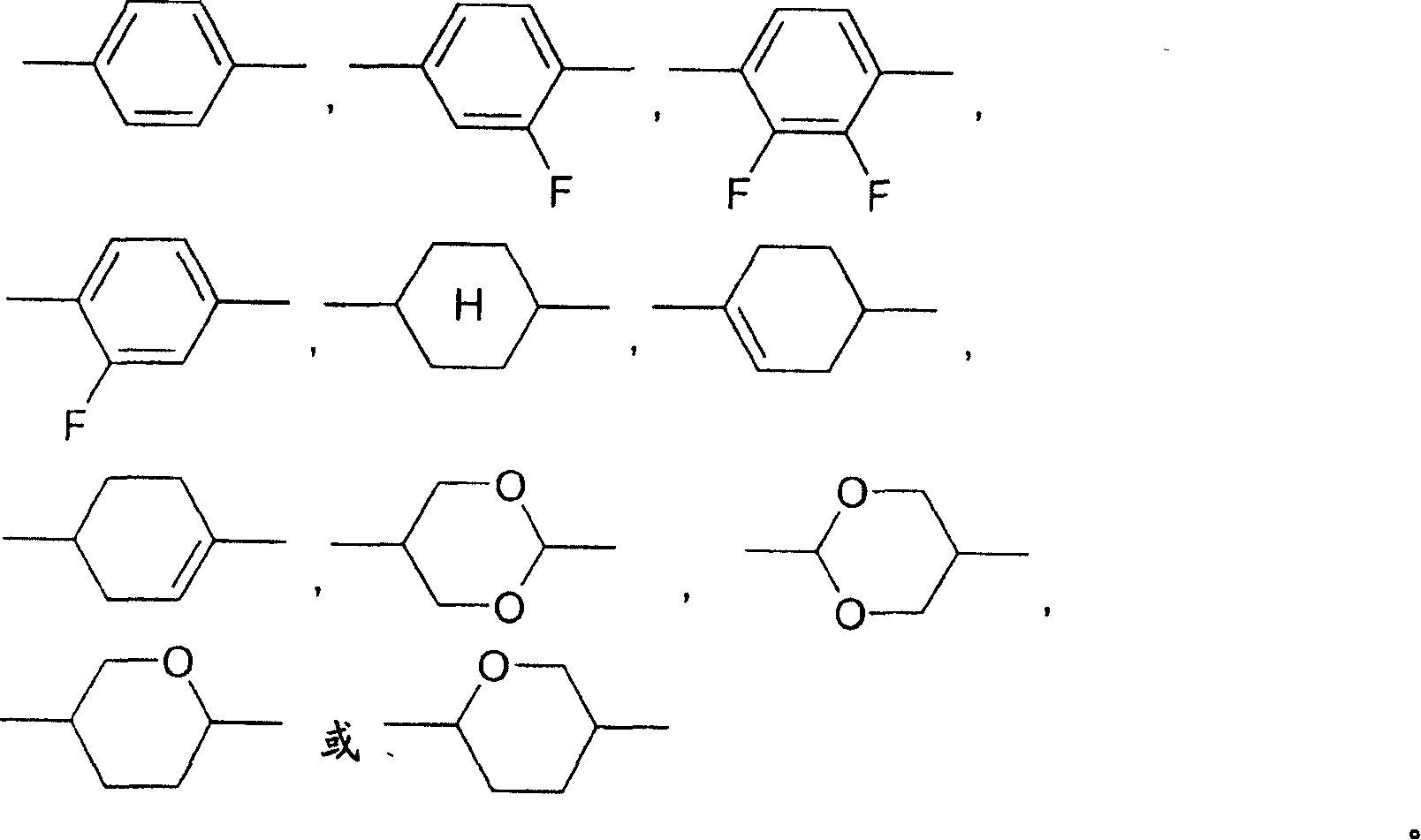
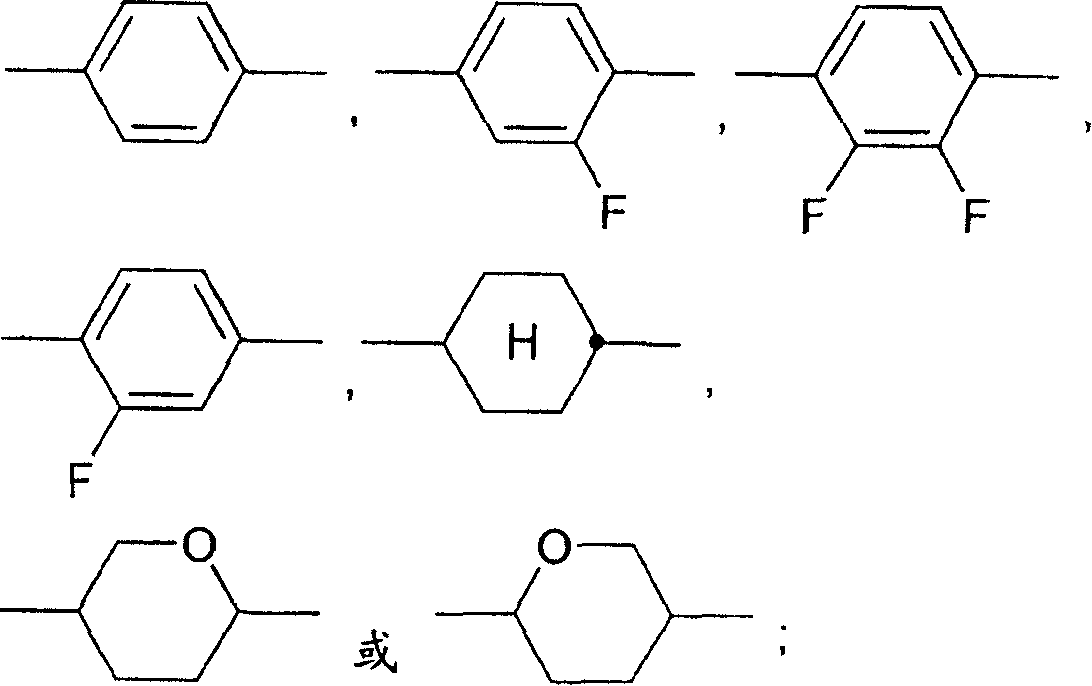
Dibenzofuran-, dibenzothiophene- and fluorene derivatives
A compound, phenylene technology, applied in the field of electro-optic display elements, can solve the problem of not giving precise details of physical or electro-optic properties, and achieve the effect of good nematic phase width
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0158]
[0159] 7.0g (22.9mmol) of silyl ether 8 was dissolved in 20ml of toluene under nitrogen, and then 5.0g (49.3mmol) of sodium carbonate, 10ml of water and 175mg (0.2mmol) of tetrakis(triphenylphosphine)palladium were added. A solution of 5.0 g (22.9 mmol) of boric acid 4 in 10 ml of absolute ethanol was slowly added to the reaction mixture at the boiling temperature. After refluxing for 5 hours and cooling, the phases separated. The aqueous phase was extracted with MTB ether. The organic phase was dried and evaporated on sodium sulfate. The residue was chromatographed on silica gel to obtain 6.5 g of silyl ether 9 (yield: 71%).
[0160]
[0161] 5.0 g (12.6 mmol) of silyl ether 9 was dissolved in 40 ml of THF, and 10 ml of dilute HCl was added. The solution was stirred at room temperature until the conversion was complete (TLC check). The solvent was then removed, and the residue was taken up in 10 ml dilute HCl solution and 20 ml MTB ether. The aqueous phase was extrac...
Embodiment 2 to 64
[0168]
[0169] Example number
Embodiment 65-128
[0171]
[0172] Example number
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com