Honokiol series derivates, preparation and use thereof
A technology of phenol derivatives and magnolia bark, applied in the field of medicinal chemistry, to achieve excellent anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
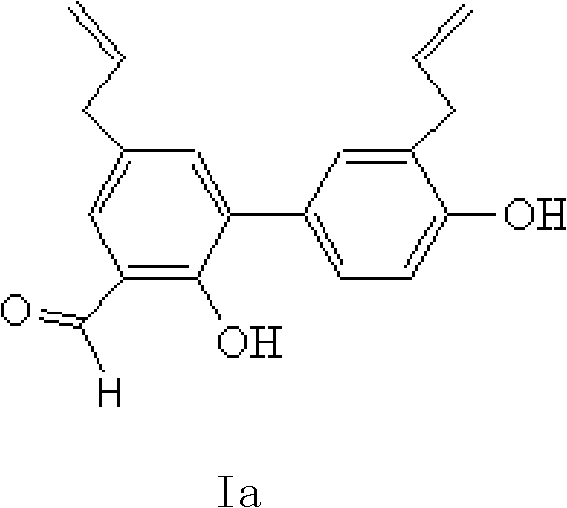
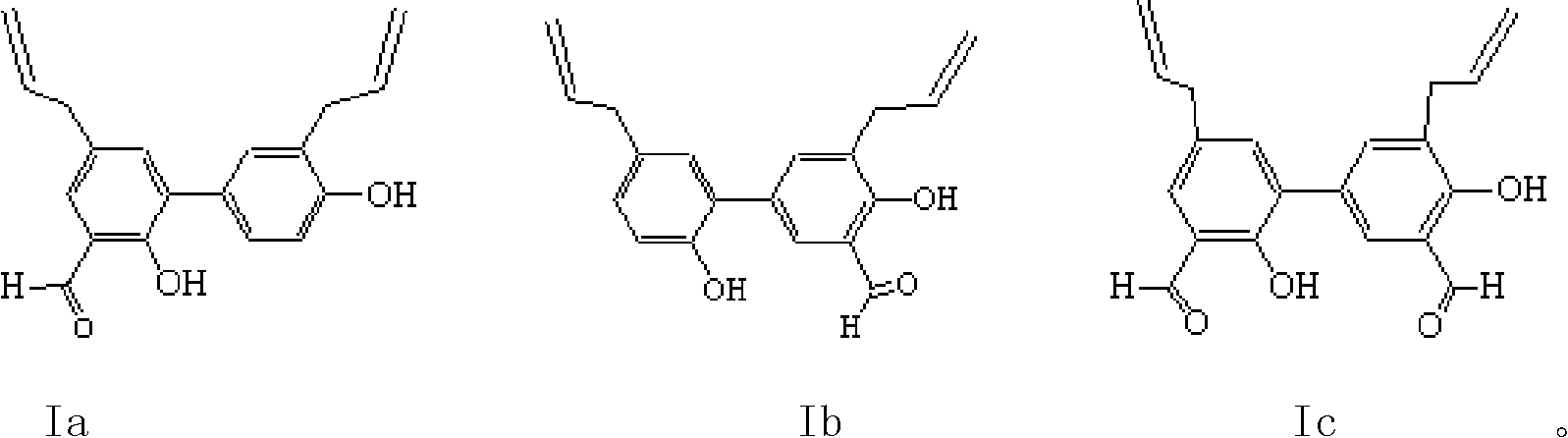
[0045] Example 1 3', the preparation of 5-diallyl-2,4'-dihydroxybiphenyl-3-aldehyde (Ia)
[0046] Dissolve 7g of sodium hydroxide in 20ml of water, add 0.266g of honokiol, use tetrabutylammonium bromide as a phase transfer catalyst, then raise the temperature of the oil bath to 65°C, slowly add 0.3ml of chloroform dropwise, and the reaction time is 2h , lower the reaction product to room temperature, adjust the pH value to 6-7 with 6mol / l hydrochloric acid, add 10ml of distilled water, stir well and extract 3 times with chloroform, combine the organic phases, wash 2 times with saturated saline, anhydrous sulfuric acid Distilled under reduced pressure after sodium drying to obtain a mixture of brown oily products.
[0047] The mixed product was separated by high-speed countercurrent chromatography, mobile phase: n-hexane-ethyl acetate, stationary phase: methanol-water, mobile phase flow rate 2ml / min, rotation speed 850rpm, sample concentration 20mg / mL, sample volume: 20ml, dete...
Embodiment 2
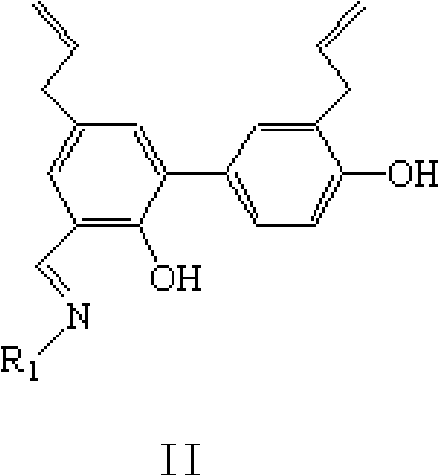
[0062] Example 2 3', the preparation of 5-diallyl-2,4'-dihydroxybiphenyl-3-oxime (IIa)
[0063] Add 0.1mmol of 3`,5-diallyl-2,4`-dihydroxybiphenyl-3-aldehyde (Ia) and 2ml of methanol into a 25ml reaction tube, place it in an EYELA synthesizer at a temperature of 25°C, and stir to make it Dissolve, add 1 mmol of hydroxylamine hydrochloride, adjust the pH to 8 with triethylamine, and stir at 25°C for 1 hour. Add 15 ml of distilled water, filter, wash with distilled water, and dry the filter cake in a vacuum oven to obtain a yellowish solid powder with a yield of 88%.
[0064] with mass spectrometry, 1 H NMR and 13 C NMR identification product, the experimental data are as follows:
[0065] 1 H NMR (400MHz, d 6 -DMSO), δ (ppm): 11.64 (s, 1H), 10.41 (s, 1H), 9.52 (s, 1H), 8.39 (s, 1H), 7.24 (q, J=2.0, 8.0Hz, 1H) , 7.22(d, J=2.0Hz, 1H), 7.14(d, J=2.0Hz, 1H), 7.06(d, J=2.0Hz, 1H), 6.86(d, J=8.0Hz, 1H), 5.91 -6.04(m, 2H), 5.00-5.11(m, 4H), 3.33(t, J=6.4, 11.2Hz, 4H);
[0066]...
Embodiment 3
[0068] The preparation of embodiment 3 honokiol derivative IIb
[0069] Add 0.1mmol of 3`,5-diallyl-2,4`-dihydroxybiphenyl-3-aldehyde (Ia) and 2ml of methanol into a 25ml reaction tube, place it in an EYELA synthesizer, control the temperature at 25°C, and stir Make it dissolve, add 0.2 mmol of cyclocitamine, adjust the pH value to 8 with triethylamine, and stir at 25°C for 1 hour. Add 15 ml of distilled water, filter the resulting precipitate, wash with distilled water, and dry the filter cake in a vacuum oven to obtain a yellowish solid powder with a yield of 85%.
[0070] with mass spectrometry, 1 H NMR and 13 C NMR identification product, the experimental data are as follows:
[0071] 1 H NMR (400MHz, CDCl 3 ), δ(ppm): 8.37(s, 1H), 7.40(q, J=1.6, 5.6Hz, 1H), 7.36(d, J=1.6Hz, 1H), 7.17(d, J=1.6Hz, 1H) , 7.01(d, J=1.6Hz, 1H), 6.86(d, J=5.6Hz, 1H), 5.94-6.09(m, 2H), 5.15-5.22(m, 2H), 3.47(d, J=4.0 Hz, 2H), 3.36(d, J=4.0Hz, 2H), 3.24(s, 1H), 1.36-1.82(m, 10H);
[0072]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com