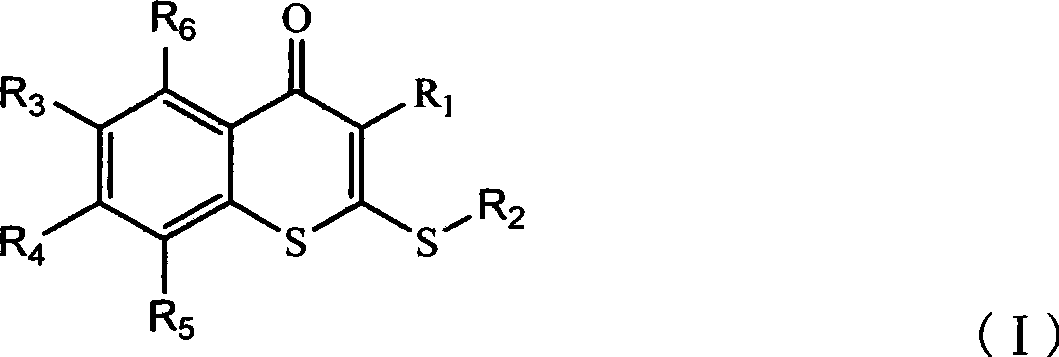
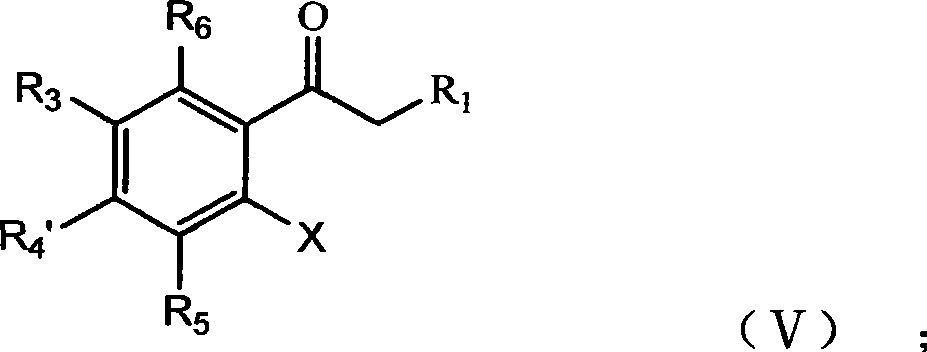
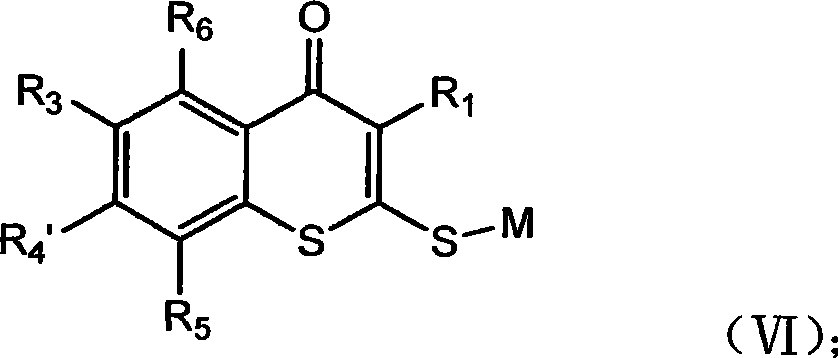
Thiochromone compound, synthetic method and application thereof in preparing antifungal medicaments
A compound, thiochromone technology, applied in the field of F, can solve the problems of increasing antibacterial activity, unable to meet broad-spectrum antifungal activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Synthesis of 1-(2,4-difluorophenyl)-2-(1H-imidazol-1-yl)-ethanone
[0092] The chemical structural formula is:
[0093]
[0094] Add imidazole (8.84g, 0.13mol), TEBA (1g), potassium carbonate (20.7g, 0.15) into a 250mL three-necked flask equipped with an electric stirrer, and then add dichloromethane (50mL) to make it suspend, and Stir for 30min. Put it in an ice bath, slowly add a solution of α-chloro-2,4-difluoroacetophenone (19.1 g, 0.01 mol) dissolved in 20 mL of DMF dropwise, and react at room temperature for 6 h after the dropping. After the reaction was completed, the solvent was recovered under reduced pressure, the residue was poured into 300 mL of ice water, the pH was adjusted to 5.0 with 1 mol / L hydrochloric acid under stirring, the layers were statically separated, and the aqueous layer was neutralized to pH = 7 with saturated sodium bicarbonate solution to obtain Pale yellow solid. The crude product was recrystallized from acetone to obtain 18.4 g of...
Embodiment 2
[0099] Synthesis of 1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazol-1-yl)ethanone
[0100] The chemical structural formula is:
[0101]
[0102] Add 1H-1,2,4-triazole (9.0g, 0.13mol), TEBA (1g, ), potassium carbonate (20.7g, 0.15mol) into a 250mL three-necked flask equipped with an electric stirrer, and then add dichloromethane (50 mL), make it suspended, and stir at room temperature for 30 min. Move it into an ice bath, slowly add a solution of α-chloro-2,4-difluoroacetophenone (19.1 g, 0.01 mol) dissolved in 20 mL of DMF dropwise, and react at room temperature for 6 h after the drop is complete. After the reaction was completed, the solvent was recovered under reduced pressure, the residue was poured into 200 mL of ice water, the pH was adjusted to 5.5 with 1 mol / L hydrochloric acid under stirring, the layers were statically separated, and the aqueous layer was neutralized to pH = 7.5 with saturated sodium bicarbonate solution to obtain Pale yellow solid. The crude product ...
Embodiment 3
[0107] Synthesis of 1-(2-fluorophenyl)-2-(1H-1,2,4-triazol-1-yl)-ethanone
[0108] The chemical structural formula is:
[0109]
[0110] Add 1H-1,2,4-triazole (9.0g, 0.13mol), TEBA (1g, ), potassium carbonate (20.7g, 0.15mol) into a 250mL three-necked flask equipped with an electric stirrer, and then add dichloromethane (50 mL), make it suspended, and stir at room temperature for 30 min. Move it into an ice bath, slowly add a solution of α-chloro-2-fluoroacetophenone (17.3 g, 0.1 mol) dissolved in 20 mL of DMF dropwise, and react at room temperature for 6 h after the drop is complete. After the reaction was completed, the solvent was recovered under reduced pressure, the residue was poured into 200 mL of ice water, the pH was adjusted to 5.5 with 1 mol / L hydrochloric acid under stirring, the layers were statically separated, and the aqueous layer was neutralized to pH = 7.5 with saturated sodium bicarbonate solution to obtain Pale yellow solid. The crude product was recr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com