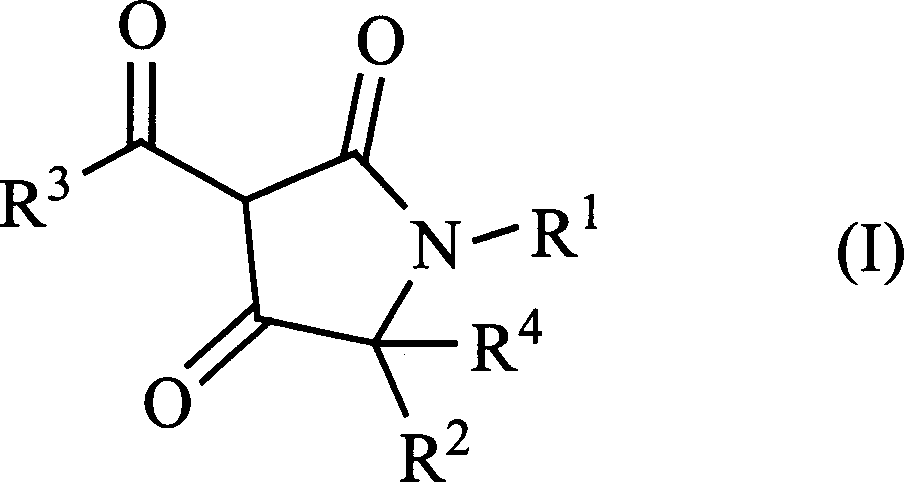
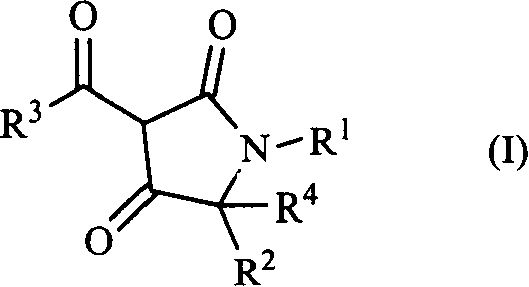
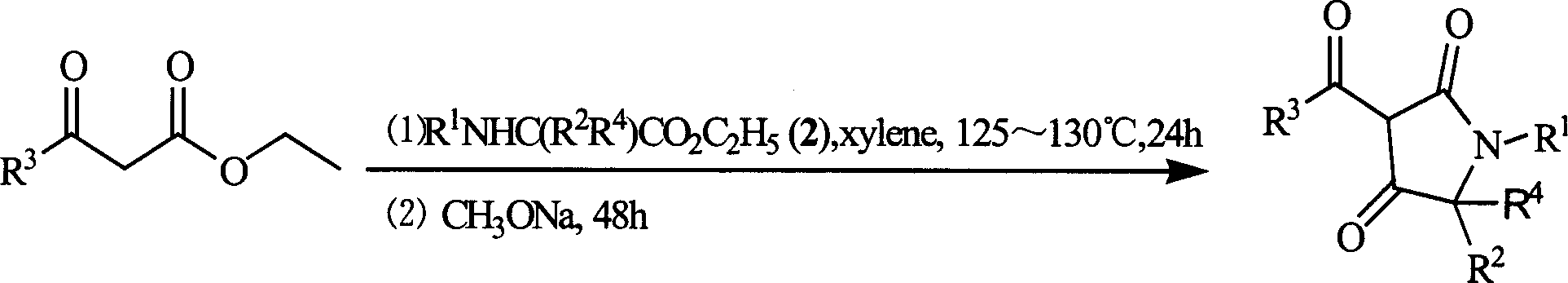3-acyl-pyrrolidine-2,4-diketo compound and herbicidal activity
A technology of acylpyrrolidine and compounds, applied in the field of 3-acylpyrrolidine-2, can solve the problems of no specific record of compound herbicidal activity, no specific record, etc., and achieve excellent selectivity, selectivity improvement, high The effect of herbicidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Synthesis of 1-isopropyl-3-(3-methylbenzoyl)pyrroline-2,4-dione
[0034] In a 150ml four-neck flask, 20 millimoles of ethyl 3-methylbenzoyl acetate and 30 millimoles of ethyl N-isopropylglycinate were stirred and reacted in 70 milliliters of benzene at 80°C for 10 hours, and then cooled to room temperature. Add 30 mmol sodium methoxide solution and stir at room temperature for 20 hours. After the solvent was removed under reduced pressure, a crude product of the target compound was obtained. The pure product is obtained by column chromatography.
Embodiment 2
[0035] Example 2: Synthesis of 1-tert-butyl-3-(3-methylbenzoyl)pyrroline-2,4-dione
[0036] In a 150ml four-neck flask, 20 millimoles of ethyl 3-methylbenzoyl acetate and 30 millimoles of ethyl N-tert-butylglycinate were stirred and reacted in 70 milliliters of benzene at 80°C for 12 hours and then cooled to room temperature. Add 37 mmol sodium methoxide solution and stir at room temperature for 20 hours. After the solvent was removed under reduced pressure, the crude product of the target compound was obtained. The pure product is obtained by column chromatography.
Embodiment 3
[0037] Example 3. Synthesis of 5-methyl-3-(2-methoxybenzoyl)pyrroline-2,4-dione
[0038] In a 150ml four-neck flask, 20 millimoles of ethyl 3-methoxybenzoyl acetate and 30 millimoles of ethyl 3-methylglycinate hydrochloride were stirred and reacted in 70 milliliters of benzene at 80°C for 46 hours, and then cooled After reaching room temperature, 41 mmol sodium methoxide solution was added and stirred at room temperature for 20 hours. After the solvent was removed under reduced pressure, a crude product of the target compound was obtained. The pure product is obtained by column chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com