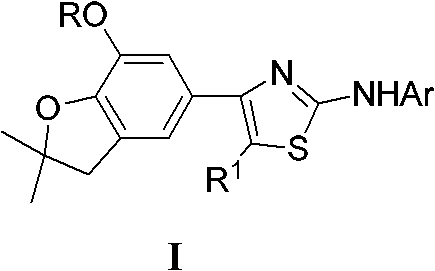
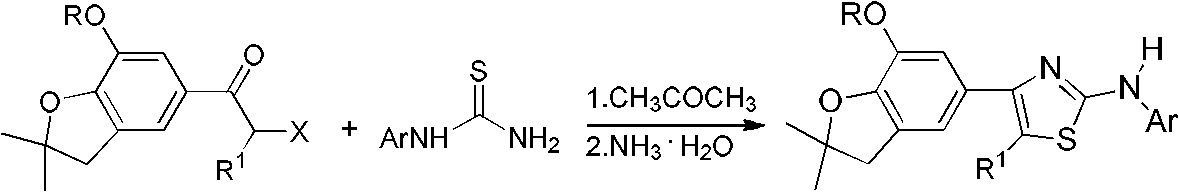5-(2-aminothiazole-4-yl) benzofuranol etheric compound with herbicidal activity and preparation method
A technology of arylaminothiazole and furan phenol ether, which is applied in the field of 5-furan phenol ether compounds, can solve the problems of high toxicity and achieve good herbicidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation reaction formula of 4-(7-methoxy-2,2-dimethyl-2,3-dihydro-5-benzofuryl)-5-methyl-2-anilinothiazole is as follows:
[0024]
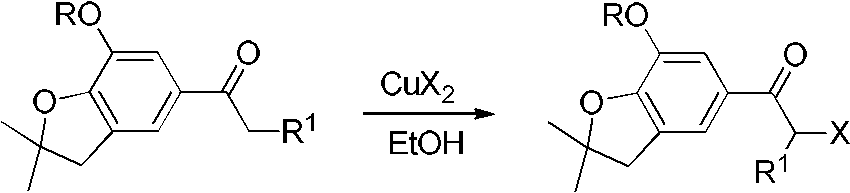
[0025] 1), the preparation of 1-(7-methoxy-2,2-dimethyl-2,3-dihydro-5-benzofuryl) acetone
[0026] Under ice-water bath, add 35.6g 7-methoxy-2,2-dimethyl-2,3-dihydrobenzofuran, 19.4g propionyl chloride, 30.7g aluminum trichloride and 150mL nitrobenzene, mix and stir After 5 hours, TLC monitored the reaction to the end point, the reaction solution was poured into ice water, separated into layers, the solvent was evaporated, and recrystallized from ethanol / water to obtain 34.3 g of light yellow solid, with a yield of 73.1%. m.p.74.4~75.5°C.
[0027] 2), the preparation of 2-bromo-1-(7-methoxy-2,2-dimethyl-2,3-dihydro-5-benzofuryl)acetone
[0028] 11.0g of 1-(7-methoxy-2,2-dimethyl-2,3-dihydro-5-benzofuryl)acetone and 100mL of ethanol were heated and stirred, and when the temperature rose to 60°C, the Add 22.4g copper bromide in...
Embodiment 2
[0031] Preparation of 4-(7-methoxy-2,2-dimethyl-2,3-dihydro-5-benzofuryl)-5-methyl-2-(2-methylanilino)thiazole
[0032] The chemical structural formula is as follows:
[0033]
[0034] The operation is the same as in Example 1, and the reaction takes 2.0h. Product R f=0.54 (developing agent: ethyl acetate:petroleum ether=1:4), dried to obtain 1.16g of light yellow solid, yield 66.3%, melting point 156-158°C. LC / MS: 381.2 (M+1). 1 HNMR (CDCl 3 , 400M) δ: 1.53(s, 6H, 2×CH 3 ), 2.30(s, 3H, thiazole ring 5-CH 3 ), 2.41 (s, 3H, CH 3 ), 3.05(s, 2H, ArCH 2 ), 3.89 (s, 3H, OCH 3 ), 6.99 (s, 2H, C 6 h 2 ), 7.05(t, 1H, J=7.6Hz, C 6 h 4 -H), 7.21(d, J=7.6Hz, 1H, C 6 h 4 6-H), 7.23(t, J=8.0Hz, 1H, C 6 h 4 5-H), 7.56(d, J=8.0Hz, 1H, C 6 h 4 3-H).
Embodiment 3
[0036] Preparation of 4-(7-methoxy-2,2-dimethyl-2,3-dihydro-5-benzofuryl)-5-methyl-2-(3-methylanilino)thiazole
[0037] The chemical structural formula is as follows:
[0038]
[0039] The operation is the same as in Example 1, and the reaction takes 2.0h. Product R f =0.51 (developing agent: ethyl acetate:petroleum ether=1:4), and dried to obtain 1.68g of light yellow solid, yield 96.0%, melting point 134-135°C. LC / MS: 381.2 (M+1). 1 H NMR (CDCl 3 , 400M) δ: 1.52(s, 6H, 2×CH 3 ), 2.34(s, 3H, thiazole ring 5-CH 3 ), 2.44 (s, 3H, CH 3 ), 3.03 (s, 2H, ArCH 2 ), 3.90 (s, 3H, OCH 3 ), 6.84~6.89 (m, 1H, C 6 h 4 4-H), 7.01(s, 2H, C 6 h 2 ), 7.09~7.24 (m, 3H, C 6 h 4 2-H, 5-H, C 6 h 4 6-H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com