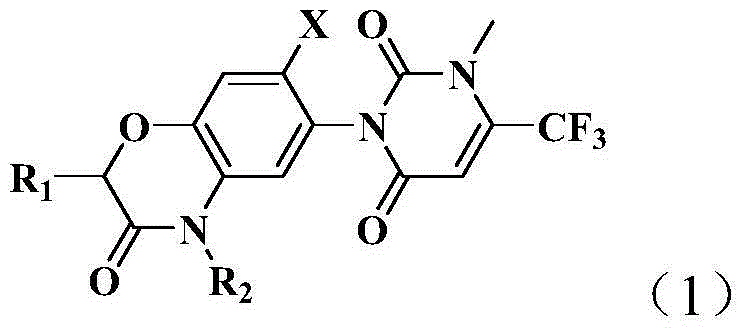
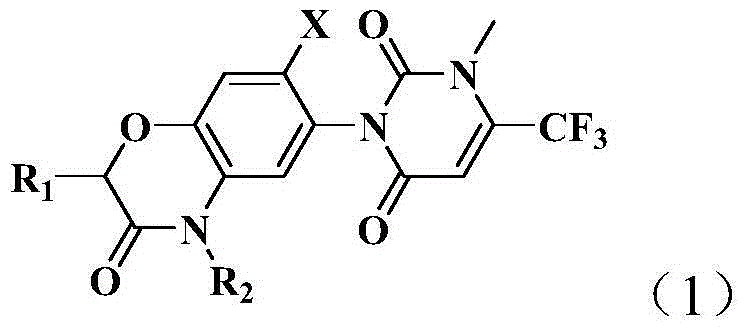
Pyrimidine diketone compounds containing benzoxazine ring and application thereof
A kind of technology of pyrimidinedione and benzoxazine ring, applied in the field of pyrimidinedione compound, can solve the problems such as unsuccessful development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
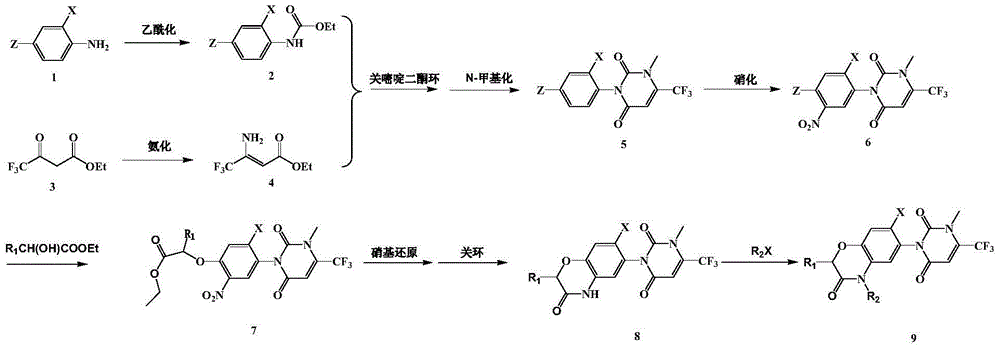
[0097] This example is used to illustrate the preparation method of the pyrimidinedione compound containing benzoxazine ring of the present invention, which is carried out according to the following synthetic route:
[0098]
[0099] 1) Synthesis of compound 2
[0100] In a 250mL round-bottomed flask, 50mmol of 2,4-difluoroaniline was dissolved in 120mL of redistilled dichloromethane and stirred, and 100mmol of pyridine was added under ice-bath conditions as an acid-binding agent. Subsequently, 50 mmol of ethyl chloroformate was dissolved in 30 mL of redistilled dichloromethane and added dropwise within 30 min. After reacting for about 1 h, it was detected by TLC that the starting material disappeared. The reaction system was neutralized to neutral with 2 mol / L hydrochloric acid, the lower organic phase was extracted, dried with anhydrous sodium sulfate, filtered, solvent removed, and compound 2 was obtained by recrystallization with petroleum ether.
[0101] 2) Synthesis...
Embodiment 2
[0133] This example is used to illustrate the preparation method of the pyrimidinedione compound containing benzoxazine ring of the present invention
[0134] According to the method in Example 1, the difference is that in step 1, 2,4-difluoroaniline is replaced by 2,4-dichloroaniline to obtain the target compounds I-21~I-40 shown in Table 1 , and its yield is shown in Table 1.
[0135] The confirmed data of compounds I-21~I-40 are as follows:
[0136] I-21: solid; m.p.87-89°C. 1 HNMR (600MHz, CDCl 3 )δ7.21(s,1H),6.65(s,1H),6.36(s,1H),4.73(s,2H),4.59(s,2H),4.21(q,J=7.2Hz,2H), 3.56(s,3H),1.23(t,J=7.2Hz,3H).EI-MS:461.16(M + ).
[0137] I-22: solid; m.p.56-57°C. 1 HNMR (600MHz, CDCl 3 )δ7.19(s,1H),6.92(s,1H),6.38(s,1H),4.66(s,2H),4.19-4.11(m,4H),3.58(s,3H),2.66(t , J=7.2Hz, 2H), 1.24(t, J=7.2Hz, 3H).EI-MS: 475.18(M + ).
[0138] I-23: solid; m.p.47-48°C. 1 HNMR (600MHz, CDCl 3 )δ7.18(s,1H),7.04(s,1H),6.38(s,1H),4.65(s,2H),4.12(q,J=7.2Hz,2H),3.92(t,J=7.2 Hz, 2H), 3.58...
Embodiment 3
[0157] This example is used to illustrate the preparation method of the pyrimidinedione compound containing benzoxazine ring of the present invention
[0158] According to the method in Example 1, the difference is that 2,4-difluoroaniline is replaced by 2-bromo-4-fluoroaniline in step 1 to obtain the target compounds I-41~I- 60, and the yields are shown in Table 1.
[0159] The confirmed data of compounds I-41~I-60 are as follows:
[0160] I-41: solid; m.p.67-68°C. 1 HNMR (600MHz, CDCl 3 )δ7.37(s,1H),6.65(s,1H),6.36(s,1H),4.73(s,2H),4.59(s,2H),4.20(q,J=7.2Hz,2H), 3.56(s,3H),1.23(t,J=7.2Hz,3H).EI-MS:505.09(M) + .
[0161] I-42: solid; m.p.69-71°C. 1 HNMR (600MHz, CDCl 3 )δ7.34(s,1H),6.92(s,1H),6.38(s,1H),4.66(s,2H),4.17-4.12(m,4H),3.58(s,3H),2.66(t , J=7.2Hz, 2H), 1.24(t, J=7.2Hz, 3H).ESI-MS: 542.1(M+Na) + .
[0162] I-43: solid; m.p.57-59°C. 1 HNMR (600MHz, CDCl 3 )δ7.33(s,1H),7.05(s,1H),6.39(s,1H),4.65(s,2H),4.12(q,J=7.2Hz,2H),3.92(t,J=7.2 Hz, 2H), 3.58(s, 3H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com