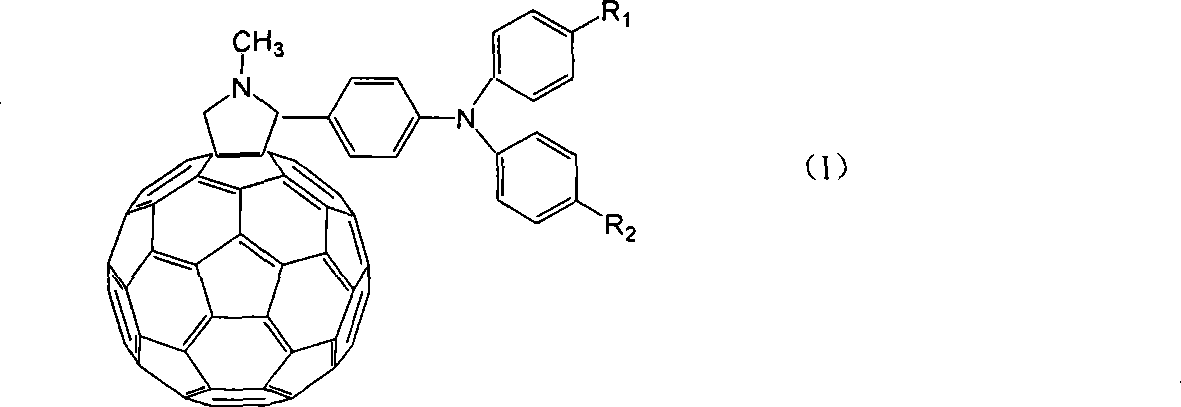
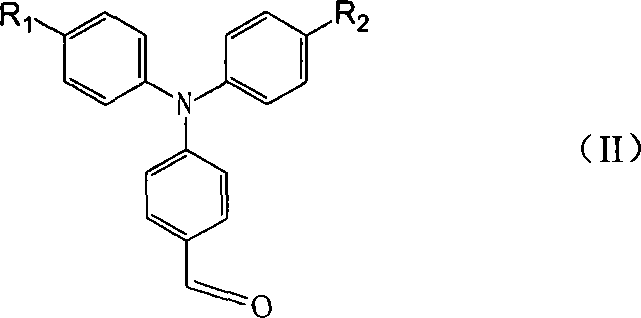
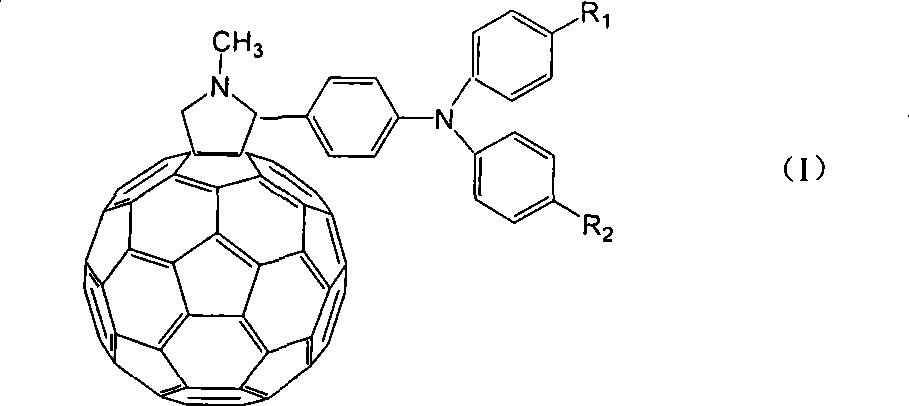
Application of C60 triarylamine derivative in solar energy battery
A derivative, C60 technology, applied in the direction of circuits, photovoltaic power generation, electrical components, etc., can solve the problems of phase separation and cluster effect, poor anti-crystallization performance, small contact area, etc., achieve simple manufacturing process, improve solubility, solve The effect of thermal decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1, the preparation of N-methyl-2-(4-N-phenyl-N-p-bromophenylamino)-fullerene pyrrolidine:
[0019] The synthetic route is as follows:
[0020]
[0021] ① Dissolve 0.546g of triphenylamine formaldehyde and 0.2mL of bromine in 20mL of dichloromethane, heat to 40°C after 1h in ice bath, stir, and react for 0.5h. After cooling, it was extracted with 10% sodium bisulfite, and the organic layer was dried over anhydrous magnesium sulfate, filtered, and recrystallized from dichloromethane / methanol. 0.608 g of a yellow solid was obtained. (Yield: 86.4%).
[0022] ②Dissolve 0.282g of the product obtained in the first step, 0.144g of fullerene, and 0.045g of sarcosine in 100mL of anhydrous toluene, heat to 100°C under the protection of nitrogen, and stir for 7h. Cooled, concentrated, separated by column chromatography, eluted with toluene:petroleum ether=1:1 (volume ratio), and dried under reduced pressure. The product was obtained in 0.143 g. (Yield: 65%). 1 H...
Embodiment 2
[0023] Embodiment 2, the preparation of N-methyl-2-(4-N-phenyl-N-p-carbonylphenylamino)-fullerene pyrrolidine:
[0024] The synthetic route is as follows:
[0025]
[0026] ① Add 14mL DMF dropwise to 16.5mL phosphorus oxychloride in ice bath, and continue ice bath for 0.5h. Dissolve 10g of triphenylamine in 100M1 1,2-dichloroethane, add to the reaction system, stir, and react at 90°C for 12h. After cooling, add a large amount of cold water, neutralize the solution with 10% sodium hydroxide solution, extract with dichloromethane, and wash the extracted solution with water, concentrate, column chromatography, and elute with toluene to obtain 5.45 g of a light yellow product. (Yield: 54.5%).
[0027] ②Dissolve 0.301g of the product obtained in the first step, 0.144g of fullerene, and 0.045g of sarcosine in 100mL of anhydrous toluene, heat to 100°C under nitrogen protection, and stir for 7h. Cool, concentrate, and separate by column chromatography, eluting with toluene, and ...
Embodiment 3
[0028] Embodiment 3, the preparation of N-methyl-2-(4-N-phenyl-N-p-nitrophenylamino)-fullerene pyrrolidine:
[0029] The synthetic route is as follows:
[0030]
[0031] ①Dissolve 0.546g triphenylamine formaldehyde with 20mL acetic anhydride, stir, add 0.37g copper nitrate, stir at room temperature for 2h, then add a mixed solution of 100mL water and 15mL chloroform, stir for 12h; stand to separate layers, and extract the two layers of the water layer with 20mL chloroform time, mixed with the crude oil layer, washed with 100 mL of water, dried, filtered, concentrated, and crystallized. 0.46 g of solid product was obtained. (Yield: 78%).
[0032] ②Dissolve 0.254g of the product obtained in the first step, 0.144g of fullerene, and 0.045g of sarcosine in 100mL of anhydrous toluene, heat to 100°C under the protection of nitrogen, and stir for 7h. Cooled, concentrated, separated by column chromatography, eluted with toluene:petroleum ether=4:1 (volume ratio), and dried under ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com