Phthalocyanine metal complex as well as preparation method and application thereof
A kind of metal complex, phthalocyanine technology, applied in the field of phthalocyanine metal complex and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1(4), 8(11), 15(18), 22(25)-tetrakis[2,4,6-tris(N,N-dimethylaminomethyl)-phenoxy]zinc phthalocyanine, Or the synthesis of four-alpha-[2,4,6-tri(N,N-dimethylaminomethyl)-phenoxy]zinc phthalocyanine comprises the following steps:
[0044] 1) With 25mmol 3-nitrophthalonitrile and 13.5mmol 2,4,6-tris(dimethylaminomethyl)-phenol as reactants, 50mL N-N-dimethylformamide as solvent A, separate After three batches of 50mmol potassium carbonate were added, under the protection of nitrogen, the reaction was stirred at 45°C for 24 hours, and monitored by thin-layer chromatography. When the 3-nitrophthalonitrile was consumed, the reaction was terminated, the reaction mixture was suction filtered, and the filtrate was collected. , evaporate the solvent to dryness, dissolve the solid with hydrochloric acid aqueous solution, remove the insolubles by suction filtration, add sodium hydroxide to the filtrate, let stand after a large amount of precipitation, suction filtration, wash the f...
Embodiment 2
[0054] 1,8(11),15(18),22(25)-tetrakis[2,4,6-tris(N,N,N-trimethylammoniomethyl)-phenoxy]phthalocyanine zinc dodecaiodide Salt, or the synthesis of four-alpha-[2,4,6-three (N,N,N-trimethylammonium methyl)-phenoxy] phthalocyanine zinc dodecaiodide salt, comprises the following steps:
[0055] 1) Prepare zinc tetrakis-α-(2,4,6-tris(N,N-dimethylaminomethyl)-phenoxy)phthalocyanine according to the method described in Example 1;
[0056] 2) Add 0.035mmol tetrakis-α-[2,4,6-tris(N,N-dimethylaminomethyl)-phenoxy]zinc phthalocyanine and 7.9mmol methyl iodide to 15mL chloroform, Stir and react at 25°C for 30 hours. After the reaction, filter with suction, rinse the filter cake with chloroform, and dry the obtained solid in vacuum to obtain 107 mg of the blue target product with a yield of 91.8%. Its structural formula is as follows:
[0057] .
[0058] The characterization data of product are as follows: IR (KBr, cm -1 ): 3010 (Ar-H); 2951, 2858 (CH 3 , CH 2 ); 1614, 1468 (benzene,...
Embodiment 3
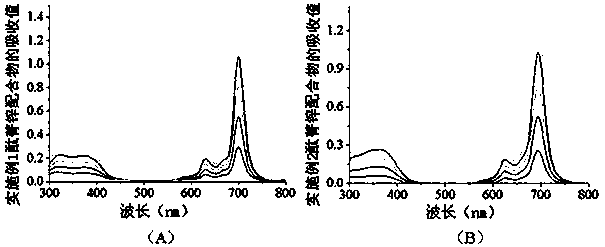
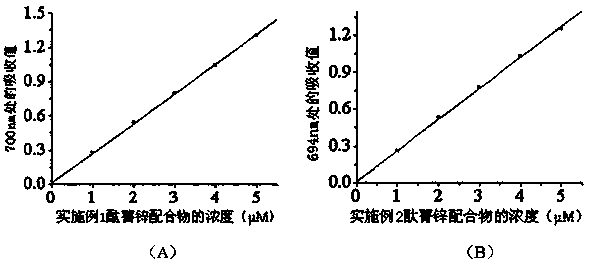
[0062]The product tetrakis-α-[2,4,6-tri(N,N-dimethylaminomethyl)-phenoxy]zinc phthalocyanine obtained in Example 1 and the product tetrakis-α-[2, 4,6-tris(N,N,N-trimethylammoniomethyl)-phenoxy]phthalocyanine zinc dodecaiodide salt wavelength scan in the range of 300-800nm, and the concentration-absorption value of each complex See the relationship diagram respectively figure 1 and figure 2 , the solvents used for the two complexes are N-N-dimethylformamide and water, respectively.
[0063] It can be seen from the figure that the zinc tetra-α-[2,4,6-tri(N,N-dimethylaminomethyl)-phenoxy]phthalocyanine has the largest The absorption wavelength is 700nm, and the molar absorptivity is 2.60×10 5 (M -1 cm -1 ); the maximum absorption wavelength of four-α-[2,4,6-tri(N,N,N-trimethylammoniomethyl)-phenoxy]phthalocyanine zinc dodecaiodide salt in water is 694nm, and the concentration It has a linear relationship with the absorption value, and the molar absorptivity is 2.52×10 5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| absorption wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com